Ethylene Oxide
- Oxirane
- ETHYLENE OXIDE
- 75-21-8
- Epoxyethane
- 1,2-Epoxyethane
- Create:2004-09-16
- Modify:2025-01-18
 Benzonatate (monomer of);
Benzonatate (monomer of);  Polidocanol (monomer of);
Polidocanol (monomer of);  Laureth-5 (monomer of) ... View More ...
Laureth-5 (monomer of) ... View More ...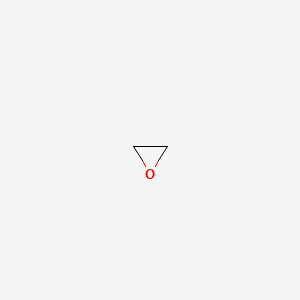
- Ethylene Oxide
- Oxide, Ethylene
- Oxirane
- Oxirane
- ETHYLENE OXIDE
- 75-21-8
- Epoxyethane
- 1,2-Epoxyethane
- Oxacyclopropane
- Dihydrooxirene
- Oxidoethane
- Oxyfume
- Ethene oxide
- Dimethylene oxide
- Amprolene
- Anprolene
- Anproline
- Aethylenoxid
- 1,2-Epoxyaethan
- Merpol
- Oxiran
- Oxyfume 12
- T-Gas
- Oxirene, Dihydro-
- Ethyleenoxide
- Oxiraan
- Ethox
- Etylenu tlenek
- FEMA No. 2433
- oxyde d'ethylene
- Rcra waste number U115
- Caswell No. 443
- Qazi-ketcham
- ETO
- NCI-C50088
- Etilene (ossido di)
- alpha,beta-Oxidoethane
- CCRIS 297
- Ethylene (oxyde d')
- ENT-26263
- ethyleneoxy
- HSDB 170
- UN 1040
- CHEBI:27561
- Oxiranyl radical
- UNII-JJH7GNN18P
- JJH7GNN18P
- EINECS 200-849-9
- EPA Pesticide Chemical Code 042301
- E.O.
- AI3-26263
- CIBA-GEIGY 9138
- DTXSID0020600
- EC 200-849-9
- epoxide or oxirane
- Sterilizing gas ethylene oxide 100%
- Oxirane; Ethylene oxide
- Oxiraan [Dutch]
- ETHYLENE OXIDE (IARC)
- ETHYLENE OXIDE [IARC]
- ETHYLENE OXIDE (MART.)
- ETHYLENE OXIDE [MART.]
- Aethylenoxid [German]
- Ethyleenoxide [Dutch]
- ethyleneoxide
- ethylenoxide
- Etylenu tlenek [Polish]
- Oxide, Ethylene
- 1,2-Epoxyaethan [German]
- 1,2-Epoxy ethane
- Ethylene (oxyde d') [French]
- Ethylene Oxide 1000 microg/mL in Triacetin
- Etilene (ossido di) [Italian]
- UN1040
- RCRA waste no. U115
- monooxirane
- Oxiranyl
- ethylene-oxide
- epoxy ethane
- Caswell no 443
- MFCD00014482
- Fema no 2433
- Epitope ID:116215
- .alpha.,.beta.-Oxidoethane
- ETHYLENE OXIDE [MI]
- DTXCID60600
- ETHYLENE OXIDE [FHFI]
- ETHYLENE OXIDE [HSDB]
- Ethylene oxide, >=99.5%
- Ethylene oxide, >=99.9%
- ALPHA, BETA-OXIDOETHANE
- CHEMBL1743219
- ETHYLENE OXIDE [WHO-DD]
- DTXSID30185475
- Ethylene oxide, purum, >=99.8%
- c0527
- AKOS009031564
- USEPA/OPP Pesticide Code: 042301
- 1ST10755-1000T
- E0647
- E0689
- E0690
- E0692
- E0693
- InChI=1/C2H4O/c1-2-3-1/h1-2H
- NS00005032
- C06548
- D03474
- Ethylene oxide Solution in Toluene, 1000ug/mL
- Q407473
- Ethylene oxide 50000 microg/mL in Dichloromethane
- Ethylene oxide 10000 microg/mL in Dimethylsulfoxide
- E O
- Ethylene oxide, or ethlene oxide with nitrogen up to a total pressure of 1Mpa (10 bar) at 50 degrees C
- Ethylene oxide, or ethlene oxide with nitrogen up to a total pressure of 1Mpa (10 bar) at 50 degrees C [UN1040] [Poison gas]
Odor Threshold Low: 257.0 [ppm]
Odor Threshold High: 690.0 [ppm]
Detection odor threshold from AIHA (mean = 420 ppm)
- Schoenflies notation
- Absorbance
- Atomic environment
- Boiling point
- Bond type
- Chemical bond
- Chemical diffusion
- Chemical shift
- Density
- Diamagnetic susceptibility
- Dielectric constant
- Diffusion
- Diffusive flux
- Dispersion
- Dissociation energy
- Electric dipole moment
- Energy
- Excess enthalpy
- Fusion temperature
- Heat of solution
- Heat of sublimation
- Hindering potential
- Internuclear distance
- Lineshape
- Magnetic anisotropy
- Magnetic permeability
- Magnetic susceptibility
- Melting temperature
- Mixing enthalpy
- Molecular dipole moment
- Molecular structure
- Moment of inertia
- Optical coefficient
- Phase diagram
- Phase equilibrium
- Phase transition
- Point group
- Refractive index
- Rotational excitation cross section
- Sound absorption
- Sound propagation
- Sound velocity
- Surface tension
- Transition enthalpy
- Vapor pressure
- Vapor-liquid equilibrium
- Vibrational mode frequency
- Virial coefficient
- Viscosity
29 99.99
44 61.78
15 54.18
14 21.78
43 15.66
29 999
44 618
15 542
14 218
43 157
Benzonatate (monomer of)
Polidocanol (monomer of)
Laureth-5 (monomer of)
Polysorbate 20 (monomer of)
- Nonoxynol-9 (monomer of)
- Polyethylene Glycol 400 (monomer of)
Tyloxapol (monomer of)
Polysorbate 80 (monomer of)
- Poloxalene (monomer of)
- Polyethylene Glycol 3350 (monomer of)
Laureth-12 (monomer of)
- Polyethylene Glycol 300 (monomer of)
- Polysorbate 40 (monomer of)
- PEG-4 diethylhexanoate (monomer of)
- Laureth-4 (monomer of)
Polixetonium Chloride (monomer of)
- Poloxamer 188 (monomer of)
Glycofurol (monomer of)
- Noneth-4 (monomer of)
Laureth-10S (monomer of)
Glycereth-7 triacetate (monomer of)
- PEG-20 Methyl Glucose Sesquistearate (monomer of)
- PEG-8 stearate (monomer of)
Polysorbate 65 (monomer of)
Propylene glycol isoceteth-3 acetate (monomer of)
- Octyldodeceth-10 (monomer of)
Tetronic 701 (monomer of)
- Ceteth-20 (monomer of)
Quaternium-52 (monomer of)
- Nonoxynol-7 (monomer of)
- Ammonium nonoxynol-4 sulfate (monomer of)
- Laureth-23 (monomer of)
- Ceteth-10 (monomer of)
PEG-150 pentaerythrityl tetrastearate (monomer of)
- Polyethylene Glycol 3500 (monomer of)
- Polyethylene Glycol 1000 (monomer of)
- Laureth-7 (monomer of)
- Polyethylene Glycol 200 (monomer of)
Menfegol (monomer of)
- Oleth-3 (monomer of)
Myreth-5 (monomer of)
Laureth-4 phosphate (monomer of)
- Isoceteth-20 (monomer of)
- Deceth-5 (monomer of)
- PEG-8 Ricinoleate (monomer of)
- Peg-100 stearate (monomer of)
- Oleth-5 (monomer of)
- Dimethicone Peg-7 isostearate (monomer of)
- Poloxamer 407 (monomer of)
- Glycereth-26 (monomer of)
- PEG-6 Methyl ether (monomer of)
Magnesium oleth sulfate (monomer of)
- Polyethylene Glycol 6000 (monomer of)
- Octoxynol-12 (monomer of)
- Polyethylene Glycol 4000 (monomer of)
- Polyethylene Glycol 800 (monomer of)
Polysorbate 85 (monomer of)
- Ceteth-6 (monomer of)
Glycereth-12 (monomer of)
- Peg-10 stearamine (monomer of)
- Bis-peg/ppg-14/14 dimethicone (monomer of)
Dihydroxypropyl peg-5 linoleammonium chloride (monomer of)
Lauryl peg/ppg-18/18 methicone (monomer of)
PEG-4 palmitamide (monomer of)
- Ceteth-10 phosphate (monomer of)
- Oleth-4 (monomer of)
- Octoxynol-11 (monomer of)
- PEG-55 Propylene Glycol Oleate (monomer of)
Di-PPG-2 myreth-10 adipate (monomer of)
- Paclitaxel Poliglumex (monomer of)
- PEG-12 Distearate (monomer of)
- Steareth-21 (monomer of)
- PEG-4 Laurate (monomer of)
PEG-4 dioleate (monomer of)
- PEG-8 dioleate (monomer of)
- Trideceth-12 (monomer of)
- Polyethylene Glycol 8000 (monomer of)
- PEG-8 Laurate (monomer of)
Pegamotecan (monomer of)
- Poloxamer 331 (monomer of)
- Polyethylene Glycol 4500 (monomer of)
- Polysorbate 21 (monomer of)
- Steareth-100 (monomer of)
- Steareth-7 (monomer of)
- Ceteth-16 (monomer of)
- Capryleth-6 (monomer of)
- Ceteth-12 (monomer of)
Bis-PEG-18 methyl ether dimethyl silane (monomer of)
- Ceteth-24 (monomer of)
- Ceteth-25 (monomer of)
- Isosteareth-10 (monomer of)
- Ceteth-15 (monomer of)
- Ceteth-30 (monomer of)
- Isolaureth-6 (monomer of)
- Methyl gluceth-10 (monomer of)
- Ceteth-5 (monomer of)
- Choleth-24 (monomer of)
- Methoxy PEG-40 (monomer of)
- Oleth-10 (monomer of)
Deceth-4 (monomer of)
- Firtecan Pegol (monomer of)
Methoxy PEG/PPG-7/3 aminopropyl dimethicone (monomer of)
- Oleth-12 (monomer of)
- Deceth-6 (monomer of)
- Laureth-10 (monomer of)
Myreth-4 (monomer of)
- Oleth-23 (monomer of)
Glycereth-7 trimethyl ether (monomer of)
Methoxy peg-22/dodecyl glycol copolymer (monomer of)
- Nonoxynol-5 (monomer of)
- Oleth-44 (monomer of)
- Laureth-11 (monomer of)
Methyl Gluceth-20 (monomer of)
Oleth-16 (monomer of)
- PEG-20 Glyceryl laurate (monomer of)
- Oleth-15 (monomer of)
- Nonoxynol-6 (monomer of)
- Oleth-40 (monomer of)
- PEG-40 stearate (monomer of)
- Oleth-25 (monomer of)
- Nonoxynol-8 (monomer of)
- Oleth-6 (monomer of)
- Pegulicianine (monomer of)
- Oleth-30 (monomer of)
- Octoxynol-5 (monomer of)
- Oleth-9 (monomer of)
- Poloxamer 237 (monomer of)
- PEG-32 Distearate (monomer of)
- Oleth-20 (monomer of)
- PEG-150 Distearate (monomer of)
- Polyethylene Glycol 1450 (monomer of)
- PEG-9 Laurate (monomer of)
- Oleth-50 (monomer of)
- PEG-150 stearate (monomer of)
- PPG-24-Glycereth-24 (monomer of)
- PEG/PPG-20/6 dimethicone (monomer of)
- Oleth-7 (monomer of)
- PEG-8 Dilaurate (monomer of)
- Oleth-8 (monomer of)
Pegcantratinib (monomer of)
- PEG-120 methyl glucose dioleate (monomer of)
- PEG-80 Sorbitan laurate (monomer of)
- PEG-3 PPG-7 ethylhexyl ether (monomer of)
- Polyethylene glycol 2000 (monomer of)
- Polyethylene Glycol 900 (monomer of)
- Steareth-4 (monomer of)
PPG-26-buteth-26 (monomer of)
- Polysorbate 81 (monomer of)
- Steareth-5 (monomer of)
- Steareth-20 (monomer of)
- Undeceth-5 (monomer of)
- Undeceth-7 (monomer of)
- Beheneth-20 (monomer of)
- Ceteth-23 (monomer of)
- Beheneth-10 (monomer of)
Beheneth-5 (monomer of)
- Davamotecan Pegadexamer (monomer of)
- Dimethicone PEG-7 phosphate (monomer of)
- Isolaureth-10 (monomer of)
- BT-051 (monomer of)
- Glycereth-5 lactate (monomer of)
- Myreth-10 (monomer of)
- Isosteareth-20 (monomer of)
- Caprylocaproyl polyoxylglycerides 8 (monomer of)
- Myreth-7 (monomer of)
- Noneth-8 (monomer of)
- Laureth-20 (monomer of)
- Ceteth-4 (monomer of)
- Nonoxynol-13 (monomer of)
- Nonoxynol-50 (monomer of)
- Methoxy PEG-16 (monomer of)
- Glycereth-7 (monomer of)
- PEG-10 Laurate (monomer of)
- PEG-10 Sorbitan laurate (monomer of)
- Nonoxynol-14 (monomer of)
- Laureth-15 (monomer of)
- PEG-20 Laurate (monomer of)
- PEG-12 Laurate (monomer of)
- Octoxynol-8 (monomer of)
- Laureth-30 (monomer of)
- Peg-7 methyl ether (monomer of)
- PEG-6 Distearate (monomer of)
- PEG-10 Stearate (monomer of)
- Laureth-6 (monomer of)
- Poloxamer 181 (monomer of)
- PEG-6 sorbitan oleate (monomer of)
PEG-15 stearamide (monomer of)
- Methoxy PEG-100 (monomer of)
- Poloxamer 184 (monomer of)
- Peg-6 stearate (monomer of)
- PEG-32 Laurate (monomer of)
- Nonoxynol-18 (monomer of)
- Polyethylene Glycol 7000 (monomer of)
- PEG-8 Distearate (monomer of)
- PEG-4 dibutyl ether (monomer of)
PEG-10 acrylate (monomer of)
PPG-2 isoceteth-20 acetate (monomer of)
- Poloxamer 124 (monomer of)
- PEG-5 Oleate (monomer of)
- PEG-10 Dioleate (monomer of)
- PPG-25-Laureth-25 (monomer of)
- Polyethylene Glycol 600 (monomer of)
- PEG-6 oleate (monomer of)
- PEG-4 Dilaurate (monomer of)
- PPG-4 Butyl ether (monomer of)
Sorbeth-12 (monomer of)
- PEG-75 Distearate (monomer of)
- PEG-9 Oleate (monomer of)
- Sodium nonoxynol-4 sulfate (monomer of)
- Steareth-16 (monomer of)
- Poloxamer 338 (monomer of)
- Poloxamer 182 (monomer of)
- Steareth-13 (monomer of)
- Steareth-30 (monomer of)
- PPG-2-Buteth-3 (monomer of)
- PPG-3-Buteth-5 (monomer of)
- Steareth-6 (monomer of)
- Steareth-40 (monomer of)
- PPG-24-Buteth-27 (monomer of)
- PPG-5-Ceteth-20 (monomer of)
- Trideceth-8 (monomer of)
- Steareth-50 (monomer of)
- PPG-28-Buteth-35 (monomer of)
Steareth-20 methacrylate (monomer of)
- PPG-33-Buteth-45 (monomer of)
- Trideceth-6 (monomer of)
- Steareth-11 (monomer of)
- Steareth-27 (monomer of)
- Steareth-15 (monomer of)
- Trideceth-9 (monomer of)
- Steareth-25 (monomer of)
- Trideceth-20 (monomer of)
- Trideceth-10 (monomer of)
- Dodoxynol-5 (monomer of)
- PEG/PPG-18/18 Dimethicone (monomer of)
- Beheneth-25 (monomer of)
- Capryleth-5 (monomer of)
- Beheneth-15 (monomer of)
- Ammonium laureth-5 sulfate (monomer of)
- Ceteth-17 (monomer of)
- Ceteth-13 (monomer of)
Beheneth-25 methacrylate (monomer of)
- Beheneth-30 (monomer of)
- Ceteth-7 (monomer of)
- Ceteth-20 phosphate (monomer of)
- Ceteth-150 (monomer of)
- Capryleth-4 (monomer of)
Ceteth-8 Phosphate (monomer of)
Choleth-10 (monomer of)
- Ceteth-18 (monomer of)
- Ceteth-40 (monomer of)
- Deceth-9 (monomer of)
- Deceth-7 (monomer of)
- Choleth-20 (monomer of)
- Laureth-16 (monomer of)
- Dimethicone PEG-10 phosphate (monomer of)
Demplatin Pegraglumer (monomer of)
- Deceth-8 (monomer of)
- Laureth-38 (monomer of)
- Glycereth-18 (monomer of)
Glycereth-18 ethylhexanoate (monomer of)
- Decyltetradeceth-30 (monomer of)
- Laureth-5 carboxylic acid (monomer of)
- Glycereth-20 (monomer of)
- Hydroxyethyl Cellulose (140 mPa.s at 5%) (monomer of)
Diethanolamine bis(C8-C18 perfluoroalkylethyl)phosphate (monomer of)
- Laureth-7 carboxylic acid (monomer of)
- Glycereth-6 laurate (monomer of)
- Laureth-25 (monomer of)
- Disteareth-100 isophorone diisocyanate (monomer of)
- Nonoxynol-20 (monomer of)
- Isosteareth-12 (monomer of)
- Laureth-40 (monomer of)
- Glycereth-31 (monomer of)
- Octoxynol-7 (monomer of)
- Isosteareth-50 (monomer of)
- Laureth-7 phosphate (monomer of)
Glycereth-7 benzoate (monomer of)
- Oleth-106 (monomer of)
- Laureth-50 (monomer of)
- Laureth-8 phosphate (monomer of)
Glycereth-7 diisononanoate (monomer of)
- PEG-10 Oleate (monomer of)
- Laureth-8 (monomer of)
- Lauryl peg-9 polydimethylsiloxyethyl dimethicone (monomer of)
- Laureth-21 (monomer of)
- PEG-11 Oleate (monomer of)
- Noneth-6 (monomer of)
- Methoxy peg-10 (monomer of)
- Myreth-12 (monomer of)
- PEG-12 diisostearate (monomer of)
- Oleth-10 phosphate (monomer of)
- Myreth-9 (monomer of)
- Nonoxynol-12 (monomer of)
- PEG-150 Dilaurate (monomer of)
- Oleth-82 (monomer of)
- Nonoxynol-100 (monomer of)
- Nonoxynol-9 phosphate (monomer of)
- PEG-150 Oleate (monomer of)
- PEG-12 dimethicone (300 cst) (monomer of)
- Oleth-11 (monomer of)
- Octoxynol-40 (monomer of)
- PEG-175 Distearate (monomer of)
- PEG-12 palmitamine (monomer of)
- Oleth-24 (monomer of)
- Octoxynol-6 (monomer of)
- PEG-20 Dilaurate (monomer of)
- PEG-15 oleamine (monomer of)
- Oleth-45 (monomer of)
- Oleth-100 (monomer of)
- PEG-20 oleate (monomer of)
- PEG-20 Dioleate (monomer of)
- PEG-12 Dilaurate (monomer of)
- Oleth-110 (monomer of)
- Peg-32 stearate (monomer of)
Peg-25 paba (monomer of)
- PEG-12 dioleate (monomer of)
- Oleth-35 (monomer of)
- PEG-4 Oleate (monomer of)
- PEG-36 Oleate (monomer of)
- PEG-12 Oleate (monomer of)
- PEG-10 oleamine (monomer of)
- Peg-40 distearate (monomer of)
- PEG-4 diisostearate (monomer of)
- Peg-12 stearate (monomer of)
- PEG-15 Oleate (monomer of)
PEG-40 sorbitan diisostearate (monomer of)
- PEG-4 oleamide (monomer of)
- PEG-14 Oleate (monomer of)
- Peg-20 glyceryl triisostearate (monomer of)
- PEG-5 oleamine (monomer of)
- PEG-40 Sorbitan stearate (monomer of)
- PEG-14 stearate (monomer of)
PEG-20 sorbitan isostearate (monomer of)
- Peg-5 stearamine (monomer of)
- PEG-6 Lauramide (monomer of)
- PEG-20 Stearate (monomer of)
- PEG-32 Dilaurate (monomer of)
- PEG-6 Dilaurate (monomer of)
- PEG-7 Oleate (monomer of)
- PEG-4 Distearate (monomer of)
- PEG-32 oleate (monomer of)
- PEG-6 oleamide (monomer of)
- PEG/PPG-20/15 dimethicone (monomer of)
- PEG-4 stearamide (monomer of)
- PEG-4 methyl ether (monomer of)
- PEG-75 Sorbitan laurate (monomer of)
- Poloxamer 185 (monomer of)
- PEG-5 Lauramide (monomer of)
- PEG-44 Sorbitan laurate (monomer of)
- Peg-8 dimethicone (monomer of)
- Poloxamer 234 (monomer of)
- PEG-6 dimethyl ether (monomer of)
- PEG-6 diisostearate (monomer of)
- PEG-8 Oleate (monomer of)
- Poloxamer 333 (monomer of)
- PEG-6 Isostearate (monomer of)
- PEG-7 dimethyl ether (monomer of)
- PEG-90 diisostearate (monomer of)
PPG-1-PEG-9 lauryl glycol ether (monomer of)
- PEG-6 Sorbitan stearate (monomer of)
- PEG-7 propylheptyl ether (monomer of)
- PEG/PPG-15/15 dimethicone (monomer of)
- Resiquimod Pegol (monomer of)
- PEG-60 Sorbitan stearate (monomer of)
- PEG-75 Dilaurate (monomer of)
- Poloxamer 183 (monomer of)
- Steareth-10 (monomer of)
- PEG-8 diisostearate (monomer of)
- PEG-75 Oleate (monomer of)
- Poloxamer 335 (monomer of)
- Steareth-8 (monomer of)
- PEG-8 dimethyl ether (monomer of)
Polixetonium (monomer of)
- Poloxamer 403 (monomer of)
- Trideceth-5 (monomer of)
- PEG-9 dimethyl ether (monomer of)
- Poloxamer 105 (monomer of)
- Ppg-1 trideceth-6 (monomer of)
- Poloxamer 334 (monomer of)
- PEG/PPG-4/12 dimethicone (monomer of)
- Poloxamer 401 (monomer of)
- Ppg-12-buteth-16 (monomer of)
- Polyethylene glycol 1600 (monomer of)
- Pentadeceth-13 (monomer of)
- Polyethylene oxide 2000000 (monomer of)
- Trideceth-11 (monomer of)
Steareth-6 stearate (monomer of)
- Poloxamer 217 (monomer of)
- Trideceth-50 (monomer of)
- Trideceth-15 (monomer of)
- Undeceth-8 (monomer of)
Sodium laureth-4 carboxylate (monomer of)
- Undeceth-9 (monomer of)
- Arachideth-95 (monomer of)
- Ammonium laureth-7 sulfate (monomer of)
- Arachideth-30 (monomer of)
- Ammonium laureth-12 sulfate (monomer of)
- Ceteth-27 (monomer of)
- Arachideth-40 (monomer of)
- Beheneth-95 (monomer of)
- Ammonium laureth-9 sulfate (monomer of)
- Ceteth-50 (monomer of)
- Beheneth-40 (monomer of)
- Ceteth-8 (monomer of)
- Capryleth-7 carboxylic acid (monomer of)
- Ceteth-60 (monomer of)
- Bis-peg/ppg-20/20 dimethicone (monomer of)
- Ceteth-9 (monomer of)
- Ceteth-11 (monomer of)
- Ceteth-80 (monomer of)
- Capryleth-7 (monomer of)
- Cetyl PEG/PPG-10/1 dimethicone (hlb 5) (monomer of)
- Ceteth-22 (monomer of)
- Deceth-20 (monomer of)
- Ceteth-100 (monomer of)
- Deceth-25 (monomer of)
- Ceteth-33 (monomer of)
- Deceth-6 phosphate (monomer of)
- Decyltetradeceth-20 (monomer of)
- Deceth-8 carboxylic acid (monomer of)
- Choleth-15 (monomer of)
- Decyltetradeceth-10 (monomer of)
- Decyltetradeceth-25 (monomer of)
- Decyltetradeceth-15 (monomer of)
- Choleth-5 (monomer of)
- Glycereth-40 oleate (monomer of)
Diethanolamine oleth-10 phosphate (monomer of)
- Decyltetradeceth-5 (monomer of)
Deceth-4 phosphate (monomer of)
- Glycereth-8 (monomer of)
- Dihydrocholeth-30 (monomer of)
- Diethanolamine laureth sulfate (monomer of)
Dimethicone peg-8 benzoate (monomer of)
- Isoceteth-25 (monomer of)
- Dimethicone peg-8 adipate (monomer of)
- Isoceteth-12 (monomer of)
Glycereth-25 PCA isostearate (monomer of)
- Isoceteth-7 (monomer of)
- Glycereth-7 lactate (monomer of)
- Isodeceth-6 (monomer of)
- Isoceteth-5 (monomer of)
- Isosteareth-15 (monomer of)
- Glycereth-80 oleate (monomer of)
- Isosteareth-5 (monomer of)
- Laureth-10 carboxylic acid (monomer of)
- Isosteareth-25 (monomer of)
- Isoceteth-10 (monomer of)
- MPEG-680 succinimidylbutanoate (monomer of)
- Myreth-20 (monomer of)
- Isosteareth-8 (monomer of)
- Isoceteth-15 (monomer of)
- Myreth-13 (monomer of)
- Nonoxynol-11 (monomer of)
- Linoleth-7 (monomer of)
- Isoceteth-30 (monomer of)
- Myreth-8 carboxylic acid (monomer of)
- Nonoxynol-6 phosphate (monomer of)
- Myreth-25 (monomer of)
- Isosteareth-16 (monomer of)
- Nonoxynol-40 (monomer of)
- Octoxynol-20 (monomer of)
- Myreth-40 (monomer of)
- Methoxy peg-25 (monomer of)
- Octoxynol-30 (monomer of)
- Octyldodeceth-25 (monomer of)
- Myreth-8 (monomer of)
- Myreth-11 (monomer of)
- Octyldodeceth-16 (monomer of)
- Oleth-20 phosphate (monomer of)
- Noneth-20 (monomer of)
- Myreth-15 (monomer of)
- Oleth-10 carboxylic acid (monomer of)
- PEG-10 glyceryl oleate (monomer of)
- Octyldodeceth-5 (monomer of)
- Myreth-30 (monomer of)
- PEG-10 dimethicone (600 cst) (monomer of)
- PEG-10 propylheptyl ether (monomer of)
- Oleth-18 (monomer of)
- Nonoxynol-25 (monomer of)
- Peg-14 dimethicone (monomer of)
- Peg-120 distearate (monomer of)
- PEG-12 oleamine (monomer of)
- Nonoxynol-44 (monomer of)
- PEG-15 palmitamine (monomer of)
- Peg-190 distearate (monomer of)
- Peg-15 stearamine (monomer of)
- Octoxynol-16 (monomer of)
- PEG-32 Dioleate (monomer of)
- PEG-5 methacrylate (monomer of)
- Peg-150 dibehenate (monomer of)
- Octyldodeceth-20 (monomer of)
- PEG-4 diacrylate (monomer of)
- PEG-6 Dioleate (monomer of)
- Peg-16 dilaurate (monomer of)
- Oleth-4 phosphate (monomer of)
- Peg-4 diheptanoate (monomer of)
- PEG-6 palmitamide (monomer of)
- PEG-175 diisostearate (monomer of)
- PEG-10 dimethyl ether (monomer of)
- PEG-4 Stearate (monomer of)
- PEG-6 stearamide (monomer of)
- PEG-18 Palmitate (monomer of)
- PEG-10 palmitamine (monomer of)
Peg-45/dodecyl glycol copolymer (monomer of)
- PEG-8 Isostearate (monomer of)
- PEG-20 Distearate (monomer of)
- PEG-12 Isostearate (monomer of)
- PEG-5 palmitamine (monomer of)
- PEG-8 oleamine (monomer of)
- Peg-200 dilaurate (monomer of)
- PEG-150 Dioleate (monomer of)
- Peg-50 stearate (monomer of)
- PEG-9 diacrylate (monomer of)
- Peg-250 distearate (monomer of)
- PEG-20 Palmitate (monomer of)
Peg-6 capramide (monomer of)
- Peg-9 distearate (monomer of)
- PEG-4 palmitamine (monomer of)
- Peg-25 stearate (monomer of)
- PEG-6 Palmitate (monomer of)
PEG/PPG-15/15 allyl ether acetate (monomer of)
- Peg-5 oleamide (monomer of)
- Peg-30 stearate (monomer of)
- PEG-7 distearate (monomer of)
- PEG/PPG-17/18 dimethicone (monomer of)
- Peg-6 glyceryl tristearate (monomer of)
PEG-40 sorbitan peroleate (monomer of)
- Peg-7 stearate (monomer of)
- PEG/PPG-18/12 dimethicone (monomer of)
- PEG-6 Laurate (monomer of)
Peg-6 caprylamide (monomer of)
- PEG-75 Dioleate (monomer of)
- PEG/PPG-22/24 dimethicone (monomer of)
PEG-6 myristamide (monomer of)
- PEG-7 diacrylate (monomer of)
- Peg-80 sorbitan palmitate (monomer of)
- Pentadeceth-15 (monomer of)
- PEG-8 methacrylate (monomer of)
- Peg-75 stearate (monomer of)
- PEG/PPG-14/4 dimethicone (monomer of)
- Pentadeceth-40 (monomer of)
- PEG-8 propylheptyl ether (monomer of)
- PEG-8 palmitamine (monomer of)
- PEG/PPG-19/19 dimethicone (monomer of)
- Pentadeceth-7 (monomer of)
- PEG/PPG-10/2 dimethicone (monomer of)
- Peg/ppg-14/7 dimethyl ether (monomer of)
- PEG/PPG-20/23 dimethicone (monomer of)
- Pentadeceth-9 (monomer of)
- PEG/PPG-125/30 Copolymer (monomer of)
- PEG/PPG-15/5 dimethicone (monomer of)
- PEG/PPG-22/40 Dimethyl ether (monomer of)
- Poloxamine 1304 (monomer of)
- PEG/PPG-20/20 dimethicone (monomer of)
- PEG/PPG-23/6 dimethicone (monomer of)
- PEG/PPG-30/10 dimethicone (monomer of)
- Polyethylene oxide 200000 (monomer of)
- PEG/PPG-25/25 dimethicone (monomer of)
- PEG/PPG-6/4 dimethicone (monomer of)
- Pentadeceth-30 (monomer of)
- Polyethylene oxide 7000000 (monomer of)
- Pentadeceth-12 (monomer of)
- Pentadeceth-10 (monomer of)
- Pentadeceth-5 (monomer of)
- Polyquaternium-10 (1000 MPA.S at 2%) (monomer of)
- Pentadeceth-20 (monomer of)
- Pentadeceth-4 (monomer of)
- Polyethylene glycol 700 (monomer of)
- Polyquaternium-10 (30000 MPA.S at 2%) (monomer of)
- Poloxamine 704 (monomer of)
- Poloxamine 908 (monomer of)
- Polyethylene oxide 1000000 (monomer of)
PPG-13-decyltetradeceth-24 (monomer of)
- Polyethylene glycol 20000 (monomer of)
- Polyethylene glycol 10000 (monomer of)
- Polyethylene oxide 5000000 (monomer of)
- Ppg-20-decyltetradeceth-10 (monomer of)
- Polyethylene oxide 100000 (monomer of)
- Polyethylene glycol 3000 (monomer of)
- Polyquaternium-10 (125 MPA.S at 2%) (monomer of)
- PPG-8-Ceteth-20 (monomer of)
- Polyquaternium-10 (400 MPA.S at 2%) (monomer of)
- Polyethylene glycol 350 (monomer of)
- Ppg-2-isodeceth-4 (monomer of)
- Sec-laureth-9 (monomer of)
- Ppg-19-buteth-19 (monomer of)
- Polyethylene oxide 600000 (monomer of)
- PPG-4-Ceteth-20 (monomer of)
Sodium laureth-4 phosphate (monomer of)
- PPG-4-Ceteth-10 (monomer of)
- Polyethylene oxide 900000 (monomer of)
- Ppg-7-buteth-10 (monomer of)
- Sodium trideceth-7 carboxylate (monomer of)
- Sodium laureth-12 sulfate (monomer of)
- Ppg-5-buteth-7 (monomer of)
- Steareth-200 (monomer of)
- Steareth-12 (monomer of)
- Sodium trideceth sulfate (monomer of)
- Sodium laureth-13 carboxylate (monomer of)
- Undeceth-40 (monomer of)
- Trideceth-13 (monomer of)
- Steareth-80 (monomer of)
- Sorbeth-12 hexaoleate (monomer of)
- Trideceth-4 carboxylic acid (monomer of)
- Trideceth-4 (monomer of)
- Undeceth-4 (monomer of)
- Trideceth-18 (monomer of)
- Undeceth-6 (monomer of)
- Trideceth-7 (monomer of)
- Undeceth-11 (monomer of)
Trideceth-8 carboxylic acid (monomer of)
- 1,2-Dimyristoyl-rac-glycero-3-methoxypolyethylene glycol 2000 (monomer of)
- Allyl peg-12 (monomer of)
Ammonium nonoxynol-30 sulfate (monomer of)
- Bis-PEG-12 dimethicone (500 MPA.S) (monomer of)
- 2-(Mpeg 2000)-N,N-ditetradecylacetamide (monomer of)
- Capryleth-25 (monomer of)
- Bis-PEG-12 dimethicone (70 MPA.S) (monomer of)
- Capryleth-9 carboxylic acid (monomer of)
- Capryleth-20 (monomer of)
- Ceteth-6 oleate (monomer of)
- Bis-peg/ppg-16/16 peg/ppg-16/16 dimethicone (monomer of)
- Ceteth-6 palmitate (monomer of)
- Caprylocaproyl polyoxylglycerides 6 (monomer of)
- Ceteth-6 stearate (monomer of)
- Dihydrocholeth-20 (monomer of)
Ceteth-7 carboxylic acid (monomer of)
- Ceteth-6 linoleate (monomer of)
- Deceth-6 carboxylic acid (monomer of)
- Dimethicone peg-8 laurate (monomer of)
- Cetyl peg/ppg-10/1 dimethicone (hlb 4) (monomer of)
- Etirinotecan Pegol Tetrahydrochloride (monomer of)
- Deceth-7 carboxylic acid (monomer of)
Ethyl acrylate/methacrylic acid/steareth-20 methacrylate copolymer (monomer of)
- Dodoxynol-7 (monomer of)
Glycereth-17 myristate (monomer of)
- Dihydrocholeth-15 (monomer of)
- Glycereth-17 caprate (monomer of)
- Glycereth-16 oleate (monomer of)
- Glycereth-5 (monomer of)
- Etirinotecan Pegol Tetratriflutate (monomer of)
- Glycereth-20 caprate (monomer of)
Glycereth-17 palmitate (monomer of)
- Glycereth-6 oleate (monomer of)
- Glycereth-17 laurate (monomer of)
- Glycereth-40 caprate (monomer of)
- Glycereth-18 benzoate (monomer of)
- Glycereth-80 (monomer of)
- Glycereth-17 oleate (monomer of)
- Glycereth-6 trioleate (monomer of)
- Glycereth-20 benzoate (monomer of)
- Glycereth-80 laurate (monomer of)
- Glycereth-30 caprate (monomer of)
- Glycereth-7 caprate (monomer of)
- Glycereth-40 laurate (monomer of)
- Hydroxyethyl cellulose (5500 mpa.S AT 2%) (monomer of)
- Glycereth-5 caprate (monomer of)
- Glycereth-7 oleate (monomer of)
- Glycereth-6 (monomer of)
- Laureth-12 succinate (monomer of)
- Glycereth-5 laurate (monomer of)
- Hydroxyethyl Cellulose (2000 mPa.s at 1%) (monomer of)
- Glycereth-6 stearate (monomer of)
- Laureth-6 phosphate (monomer of)
- Glycereth-80 caprate (monomer of)
- Linoleth-20 (monomer of)
- Glycereth-7 stearate (monomer of)
- Laureth-9 phosphate (monomer of)
- Laureth-4 carboxylic acid (monomer of)
- Meroxapol 251 (monomer of)
- Laureth-10 phosphate (monomer of)
- Lysine, zilucoplan- (monomer of)
- Linoleth-25 (monomer of)
- Meroxapol 255 (monomer of)
- Laureth-11 carboxylic acid (monomer of)
- Meroxapol 105 (monomer of)
- Magnesium laureth-8 sulfate (monomer of)
- Meroxapol 312 (monomer of)
- Laureth-8 carboxylic acid (monomer of)
- Meroxapol 172 (monomer of)
- Meroxapol 108 (monomer of)
- Methoxy peg-12 retinamide (monomer of)
- Meroxapol 171 (monomer of)
- Meroxapol 258 (monomer of)
- Meroxapol 174 (monomer of)
Methoxy-PEG-7 rutinyl succinate (monomer of)
- Meroxapol 178 (monomer of)
- Meroxapol 314 (monomer of)
- Meroxapol 252 (monomer of)
- Oleth-3 carboxylic acid (monomer of)
- Meroxapol 254 (monomer of)
Methoxy PEG-9 methacrylate (monomer of)
- Methoxy peg maleimide (20000 MW) (monomer of)
- Peg-10 campestanol (monomer of)
- Meroxapol 311 (monomer of)
- Mpeg 5000 lysine (monomer of)
- Methoxy peg-20 (monomer of)
- Peg-10 capramine (monomer of)
- Methoxy peg maleimide (30000 MW) (monomer of)
- Mpeg 6000 acetyl lysine (monomer of)
- Mpeg-20000 lysine (monomer of)
PEG-10 stigmasteryl ether (monomer of)
- Myreth-6 phosphate (monomer of)
- Mpeg5000 methionine (monomer of)
- Myreth-9 phosphate (monomer of)
- Peg-15 capramine (monomer of)
- Nonoxynol-10 phosphate (monomer of)
- Myreth-5 carboxylic acid (monomer of)
- N6-(Mpeg-12000)-lysine (monomer of)
- Peg-15 diallyl ether (monomer of)
- Octoxynol-20 carboxylic acid (monomer of)
- Myreth-7 carboxylic acid (monomer of)
- PEG-10 beta-SITOSTERYL ETHER (monomer of)
- Peg-15 myristamine (monomer of)
- Oleth-5 phosphate (monomer of)
- Octoxynol-35 (monomer of)
- Peg-10 campesterol (monomer of)
- Peg-15 stearate (monomer of)
- Oleth-6 carboxylic acid (monomer of)
- Octoxynol-55 (monomer of)
- PEG-10 glyceryl trioleate (monomer of)
- Peg-16 oleate (monomer of)
- Oleth-7 phosphate (monomer of)
- Peg-10 caprylamine (monomer of)
- Peg-10 myristamine (monomer of)
- Peg-18 stearate (monomer of)
- Peg-10 diallyl ether (monomer of)
- Peg-10 eicosyl ether (monomer of)
- Peg-12 caprylamine (monomer of)
- PEG-20 glyceryl trioleate (monomer of)
- Peg-10 glyceryl stearate (monomer of)
- Peg-10 linoleamine (monomer of)
- PEG-12 Glyceryl laurate (monomer of)
- Peg-200 stearate (monomer of)
- Peg-10 lauramine (monomer of)
- Peg-12 capramine (monomer of)
- Peg-12 lauramine (monomer of)
- PEG-23 Glyceryl laurate (monomer of)
- Peg-10 stigmastanol (monomer of)
- Peg-12 glyceryl dimyristate (monomer of)
- Peg-12 myristamine (monomer of)
- PEG-30 Glyceryl laurate (monomer of)
- Peg-12 stearamine (monomer of)
- Peg-12 linoleamine (monomer of)
- PEG-14 PPG-7 ethylhexyl ether (monomer of)
- Peg-4 linoleamide (monomer of)
- Peg-120 glyceryl stearate (monomer of)
- Peg-120 stearate (monomer of)
- Peg-15 caprylamine (monomer of)
- Peg-4 myristamine (monomer of)
- PEG-14 propylheptyl ether (monomer of)
- PEG-15 glyceryl oleate (monomer of)
- Peg-15 glyceryl stearate (monomer of)
- PEG-4 propylheptyl ether (monomer of)
- Peg-15 linoleamine (monomer of)
- PEG-20 glyceryl oleate (monomer of)
- Peg-15 lauramine (monomer of)
- Peg-5 caprylamide (monomer of)
- Peg-20 behenate (monomer of)
- Peg-23 oleate (monomer of)
- Peg-20 myristate (monomer of)
- Peg-5 ethylhexanoate (monomer of)
- Peg-20 dibehenate (monomer of)
- PEG-25 glyceryl trioleate (monomer of)
- Peg-23 stearate (monomer of)
- Peg-5 linoleamine (monomer of)
- Peg-20 isostearate (monomer of)
- Peg-35 stearate (monomer of)
- Peg-25 glyceryl isostearate (monomer of)
- Peg-5 myristamide (monomer of)
- Peg-20 sorbitan stearate (monomer of)
- PEG-40 glyceryl trioleate (monomer of)
- PEG-30 Glyceryl oleate (monomer of)
- Peg-5 stearate (monomer of)
- Peg-200 glyceryl stearate (monomer of)
- Peg-5 acrylate (monomer of)
- PEG-30 glyceryl trioleate (monomer of)
- Peg-6 linoleamide (monomer of)
- PEG-25 glyceryl oleate (monomer of)
- Peg-5 brassicasterol (monomer of)
- Peg-4 capramine (monomer of)
- Peg-60 monolaurate (monomer of)
- Peg-25 propylene glycol stearate (monomer of)
- Peg-5 caprylamine (monomer of)
- Peg-4 lauramine (monomer of)
- Peg-8 capramine (monomer of)
- Peg-36 stearate (monomer of)
- Peg-5 myristamine (monomer of)
- Peg-4 linoleamine (monomer of)
- Peg-8 glyceryl isostearate (monomer of)
- Peg-4 caprylamine (monomer of)
- PEG-5 propylheptyl ether (monomer of)
- Peg-4 stearamine (monomer of)
- Peg-8 myristamine (monomer of)
- Peg-4 oleamine (monomer of)
- PEG-6 PPG-7 ethylhexyl ether (monomer of)
- Peg-45 stearate (monomer of)
- Peg-8 undecylenate (monomer of)
- Peg-5 campesterol (monomer of)
- Peg-8 caprate (monomer of)
- Peg-5 campestanol (monomer of)
- Peg-9 isostearate (monomer of)
- Peg-5 capramine (monomer of)
- Peg-8 linoleamine (monomer of)
- Peg-5 capramide (monomer of)
- Peg-9 polydimethylsiloxyethyl dimethicone (monomer of)
- Peg-5 linoleamide (monomer of)
- Peg-8 myristate (monomer of)
- PEG-5 glyceryl oleate (monomer of)
- Peg/ppg-105/5 copolymer (monomer of)
- Peg-5 methyl ether (monomer of)
- Peg-80 stearate (monomer of)
- PEG-5 glyceryl trioleate (monomer of)
- Peg/ppg-17/6 copolymer (monomer of)
- PEG-50 glyceryl trioleate (monomer of)
- PEG-9 propylheptyl ether (monomer of)
- Peg-5 lauramine (monomer of)
- Peg/ppg-36/41 dimethyl ether (monomer of)
- Peg-7 laurate (monomer of)
- Peg/ppg-116/66 copolymer (monomer of)
- Peg-5 palmitamide (monomer of)
- Peg/ppg-9/2 dimethyl ether (monomer of)
- Peg-8 caprylate (monomer of)
- Peg/ppg-30/160 copolymer (monomer of)
- Peg-5 stearamide (monomer of)
- Peginesatide linker (monomer of)
- Peg-8 lauramine (monomer of)
- Peg/ppg-35/40 dimethyl ether (monomer of)
- Peg-5 stigmastanol (monomer of)
- Polyethylene glycol 11000 (monomer of)
PEG-9 diglycidyl ether (monomer of)
- Peg/ppg-50/40 dimethyl ether (monomer of)
- Peg-55 stearate (monomer of)
- Polyethylene glycol 250 (monomer of)
- Peg/ppg-17/4 dimethyl ether (monomer of)
- Polyethylene glycol 2500 (monomer of)
- PEG-6 propylheptyl ether (monomer of)
- Polyethylene oxide 300000 (monomer of)
- Peg/ppg-3/6 dimethyl ether (monomer of)
- Polyethylene oxide 400000 (monomer of)
- PEG-60 glyceryl trioleate (monomer of)
- Ppg-12-peg-50 (monomer of)
- Peg/ppg-55/28 dimethyl ether (monomer of)
Polyglyceryl-2-PEG-4 stearate (monomer of)
- Peg-60 stearate (monomer of)
- Ppg-33-buteth-35 (monomer of)
- Peg/ppg-7/12 dimethyl ether (monomer of)
- Potassium trideceth-7 phosphate (monomer of)
- Peg-8 arachidate (monomer of)
- Ricinoleth-40 (monomer of)
- Pentadeceth-8 (monomer of)
- Ppg-2-steareth-9 (monomer of)
- Peg-8 caprylamine (monomer of)
- Sec-laureth-12 (monomer of)
- Polyethylene oxide 8000000 (monomer of)
- Sec-laureth-15 (monomer of)
- Peg-8 palmitate (monomer of)
- Sec-laureth-7 (monomer of)
- Sec-laureth-30 (monomer of)
- Sec-laureth-40 (monomer of)
- Peg-8 stearamine (monomer of)
- Sec-myreth-15 (monomer of)
- Sec-laureth-50 (monomer of)
- Sec-myreth-20 (monomer of)
- Peg-9 dimethacrylate (monomer of)
- Sec-myreth-40 (monomer of)
- Sec-myreth-12 (monomer of)
- Sec-myreth-5 (monomer of)
- Peg-9 stearate (monomer of)
- Sec-myreth-7 (monomer of)
- Sec-myreth-30 (monomer of)
- Sec-myreth-9 (monomer of)
- Peg-90 stearate (monomer of)
- Sec-trideceth-40 (monomer of)
- Sec-trideceth-12 (monomer of)
- Sec-trideceth-15 (monomer of)
- Peg/ppg-27/14 dimethyl ether (monomer of)
- Sec-trideceth-7 (monomer of)
- Sec-trideceth-30 (monomer of)
- Sec-trideceth-9 (monomer of)
- Peg/ppg-52/32 dimethyl ether (monomer of)
- Sodium laureth-5 sulfate (monomer of)
- Sec-trideceth-50 (monomer of)
- Sodium laureth-11 carboxylate (monomer of)
- Pentadeceth-11 (monomer of)
Sodium undeceth-5 carboxylate (monomer of)
- Sec-undeceth-12 (monomer of)
- Sodium laureth-6 carboxylate (monomer of)
- Polyethylene glycol 12000 (monomer of)
- Steareth-17 (monomer of)
- Sodium laureth-5 carboxylate (monomer of)
- Sodium laureth-8 sulfate (monomer of)
- Polyethylene oxide 4000000 (monomer of)
- Steareth-23 (monomer of)
- Sodium trideceth-8 carboxylate (monomer of)
- Sodium undeceth-6 carboxylate (monomer of)
- Ppg-12 capryleth-18 (monomer of)
- Steareth-6 palmitate (monomer of)
- Sorbeth-12 hexaisostearate (monomer of)
- Sorbeth-12 hexalaurate (monomer of)
- Ppg-12-peg-65 (monomer of)
- Steareth-60 (monomer of)
- Steareth-12 stearate (monomer of)
- Steareth-33 (monomer of)
- Ppg-2-ceteth-9 (monomer of)
- Trideceth-30 (monomer of)
- Steareth-6 oleate (monomer of)
- Steareth-6 linoleate (monomer of)
- Ppg-40-peg-60 (monomer of)
- Trideceth-40 (monomer of)
- Trideceth-23 (monomer of)
- Steareth-60 cetyl ether (monomer of)
- Sec-laureth-20 (monomer of)
- Undeceth-20 (monomer of)
- Trideceth-7 carboxylic acid (monomer of)
Steareth-7 carboxylic acid (monomer of)
- Sec-laureth-5 (monomer of)
- Undeceth-6 carboxylic acid (monomer of)
- Undeceth-10 (monomer of)
- Trideceth-6 phosphate (monomer of)
- Sec-myreth-50 (monomer of)
- Undeceth-30 (monomer of)
- Undeceth-15 (monomer of)
- Sec-pentadeceth-12 (monomer of)
- Undeceth-8 carboxylic acid (monomer of)
- Sec-trideceth-20 (monomer of)
- Sec-trideceth-5 (monomer of)
- Steareth-10 phosphate (monomer of)
- Steareth-22 (monomer of)
- Steareth-5 stearate (monomer of)
- Tocofersolan diester (monomer of)
- Trideceth-10 phosphate (monomer of)
- Undeceth-12 (monomer of)
Bis-PEG-10 dimethicone/dimer dilinoleate copolymer (monomer of)
- Caprylocaproyl polyoxylglycerides 4 (monomer of)
- alpha-SUCCINIMIDYLOXY CARBONYL-omega-METHOXY, POLYOXYETHYLENE MW 5000 (monomer of)
- Alanine N-butyl mpeg 30000 (monomer of)
- Cysteine, (pegulicianine)- (monomer of)
- Ceteth-6 palmitoleate (monomer of)
- Ammonium nonoxynol-9 sulfate (monomer of)
- Bis(cysteinyl maleimide) peg 20000 (monomer of)
- DI(Peg-7) linoleoyl glycerides (monomer of)
- Ceteth-60 myristyl glycol (monomer of)
- Cetyl hydroxyethylcellulose (350000 MW) (monomer of)
- C8-10/propylene glycol/peg-22/decylene glycol ether (monomer of)
- Dimethicone peg-7 lactate (monomer of)
- Cetyl peg/ppg-10/1 dimethicone (hlb 2) (monomer of)
- Cysteine, bms-962476- (monomer of)
- Capryleth-6 carboxylic acid (monomer of)
- Dimethicone peg-7 phthalate (monomer of)
- Cysteine, lulizumab pegol- (monomer of)
- Decyltetradeceth-200 behenate (monomer of)
- Dimethicone peg-7 undecylenate (monomer of)
- Dimethicone peg-8 phosphate (monomer of)
- Diceteth-4 phosphate sodium (monomer of)
- Dimethicone peg-15 acetate (monomer of)
- Dimethicone peg-8 isostearate (monomer of)
- Glycereth-16 palmitoleate (monomer of)
- Diethylaminoethyl peg-5 oleate (monomer of)
- Dimethicone peg-7 succinate (monomer of)
- Dimethicone peg-8 phthalate (monomer of)
- Glycereth-17 caprylate (monomer of)
- Dimethicone peg-8 succinate (monomer of)
- Glycereth-16 stearate (monomer of)
- Disteareth-4 phosphate sodium (monomer of)
- Glycereth-20 caprylate (monomer of)
- Disteareth-75 isophorone diisocyanate (monomer of)
- Glycereth-20 linoleate (monomer of)
- Glycereth-17 linoleate (monomer of)
- Glycereth-20 palmitate (monomer of)
- Glycereth-16 palmitate (monomer of)
- Glycereth-6 linoleate (monomer of)
- Glycereth-20 myristate (monomer of)
- Glycereth-30 palmitate (monomer of)
- Glycereth-17 stearate (monomer of)
- Glycereth-6 palmitate (monomer of)
- Glycereth-30 caprylate (monomer of)
- Glycereth-40 caprylate (monomer of)
- Glycereth-26 caprylate (monomer of)
- Glycereth-6 tricaprylate (monomer of)
- Glycereth-40 palmitate (monomer of)
- Glycereth-40 myristate (monomer of)
- Glycereth-30 myristate (monomer of)
- Glycereth-7 linoleate (monomer of)
- Glycereth-5 caprylate (monomer of)
- Glycereth-5 palmitate (monomer of)
- Glycereth-40 linoleate (monomer of)
- Glycereth-80 linoleate (monomer of)
- Glycereth-5 linoleate (monomer of)
- Glycereth-6 trilinoleate (monomer of)
- Glycereth-5 myristate (monomer of)
- Glycereth-80 palmitate (monomer of)
- Glycereth-6 triisostearate (monomer of)
- Glycereth-6 tripalmitate (monomer of)
- Glycereth-6 caprylate (monomer of)
- Hydroxyethyl cellulose (1800 mpa.S AT 2%) (monomer of)
- Glycereth-6 trimyristate (monomer of)
- Glycereth-7 1,3-diisostearate (monomer of)
- Glycereth-6 myristate (monomer of)
- Hydroxyethyl cellulose (280 mpa.S AT 5%) (monomer of)
- Glycereth-7 glycolate (monomer of)
- Glycereth-7 caprylate (monomer of)
- Glycereth-6 tricaprate (monomer of)
- Hydroxyethyl cellulose (4000 mpa.S AT 1%) (monomer of)
- Glycereth-7 myristate (monomer of)
- Glycereth-7 palmitate (monomer of)
- Glycereth-6 trilaurate (monomer of)
- Hydroxyethyl cellulose (6500 mpa.S AT 2%) (monomer of)
- Glycereth-80 caprylate (monomer of)
- Hydroxyethyl cellulose (1500 mpa.S AT 1%) (monomer of)
- Glycereth-7 1,2-diisostearate (monomer of)
- Isoceteth-10 stearate (monomer of)
- Glycereth-80 myristate (monomer of)
- Hydroxyethyl cellulose (280 mpa.S AT 2%) (monomer of)
- Glycereth-8 hydroxystearate (monomer of)
- Laureth-5 butyl ether (monomer of)
- Glycereth-80 stearate (monomer of)
- Methoxy peg-10 maleate (monomer of)
- Isosteareth-10 stearate (monomer of)
- Magnesium 1-laureth-3 sulfosuccinate (monomer of)
- Hydroxyethyl cellulose (100 mpa.S AT 2%) (monomer of)
- Mpeg 30000 butanoyl N-leucine (monomer of)
- Linoleth-7 carboxylic acid (monomer of)
- Mpeg 5000-dspe free acid (monomer of)
- Hydroxyethyl cellulose (3000 mpa.S AT 1%) (monomer of)
- Mpeg-propionaldehyde (5000 MW) (monomer of)
- Monoceteth-4 phosphate sodium (monomer of)
- Mpeg 6000 acetyl serine (monomer of)
- Hydroxyethyl cellulose (5000 mpa.S AT 1%) (monomer of)
- N-(Mpeg-5000)-alanine (monomer of)
- Mpeg 40000 phosphoric acid (monomer of)
- Noneth-6 carboxylic acid (monomer of)
- Laureth-6 carboxylic acid (monomer of)
- N-Propyl mpeg 20000 glycine (monomer of)
- Mpeg 5000-spa-N6-lysine (monomer of)
- Nonoxynol-4 phosphate (monomer of)
- Methoxy peg-450 maleimide (monomer of)
- Noneth-8 carboxylic acid (monomer of)
- Mpeg-400-gly-neu-galnac-thr (monomer of)
- Peg-10 tetracosanyl ether (monomer of)
- Mpeg 30000 butanoyl N6-lysine (monomer of)
- Oleth-7 carboxylic acid (monomer of)
- Mpeg-leucine (30000 MW) (monomer of)
- Peg-12 glyceryl 1,2-distearate (monomer of)
- Mpeg-20000-maleimide-cysteine (monomer of)
- Peg-10 hexatriacontanyl ether (monomer of)
- N-(Mpeg-12000)-cysteine (monomer of)
- Peg-140 glyceryl tristearate (monomer of)
- Mpeg-isoleucine (20000 MW) (monomer of)
- Peg-10 octatriacontanyl ether (monomer of)
- N3-(Mpeg-12000)-histidine (monomer of)
- Peg-20 glyceryl isostearate (monomer of)
- Mpeg-propyl-amino-1-oxo-propyl-maleimide (40000 MW) (monomer of)
- Peg-10 tetracontanyl ether (monomer of)
- O-(Mpeg40000-glyc)ser (monomer of)
- Peg-20 glyceryl stearate (monomer of)
- N-(Mpeg-5000)-leucine (monomer of)
- Peg-10 triacontanyl ether (monomer of)
- PEG-10 beta-SITOSTEROL (monomer of)
- Peg-20 stigmasteryl ether (monomer of)
- Peg-10 brassicasterol (monomer of)
- Peg-15 hydroxystearate (monomer of)
- Peg-10 glyceryl isostearate (monomer of)
- Peg-23 glyceryl 1,3-distearate (monomer of)
- Peg-10 campesteryl ether (monomer of)
- Peg-15 stearmonium chloride (monomer of)
- Peg-10 glyceryl triisostearate (monomer of)
- Peg-30 campesteryl ether (monomer of)
- Peg-10 dotriacontanyl ether (monomer of)
- Peg-16 campesteryl ether (monomer of)
- Peg-10 hexacosanyl ether (monomer of)
- Peg-32 glyceryl 1-palmitate (monomer of)
- Peg-10 glyceryl tristearate (monomer of)
- Peg-16 stigmasteryl ether (monomer of)
- Peg-10 tetratriacontanyl ether (monomer of)
- Peg-4 glyceryl 1,2-distearate (monomer of)
- Peg-10 octacosanyl ether (monomer of)
- Peg-18 glyceryl oleate/caprate (monomer of)
- Peg-12 glyceryl 1,3-distearate (monomer of)
- Peg-40 campesteryl ether (monomer of)
- Peg-12 glyceryl linoleate (monomer of)
- Peg-20 campesteryl ether (monomer of)
- Peg-15 glyceryl isostearate (monomer of)
- Peg-40 hexacosanyl ether (monomer of)
- Peg-15 12-hydroxystearic acid (monomer of)
- Peg-20 glyceryl tristearate (monomer of)
- PEG-16 beta-SITOSTERYL ETHER (monomer of)
- Peg-40 octatriacontanyl ether (monomer of)
- Peg-15 DI(hydroxystearate) (monomer of)
- Peg-200 glyceryl 1-stearate (monomer of)
- PEG-20 beta-SITOSTERYL ETHER (monomer of)
- PEG-5 beta-SITOSTEROL (monomer of)
- Peg-15 glyceryl tristearate (monomer of)
- Peg-23 glyceryl 1,2-distearate (monomer of)
- Peg-200 glyceryl 1-palmitate (monomer of)
- Peg-60 glyceryl isostearate (monomer of)
- Peg-18 glyceryl oleate/laurate (monomer of)
- Peg-25 campesteryl ether (monomer of)
- Peg-30 dipolyhydroxystearate (4000 MW) (monomer of)
- Peg-7 dilinoleoyl glycerides (monomer of)
- Peg-18 glyceryl oleate/oleate (monomer of)
- Peg-25 stigmasteryl ether (monomer of)
- Peg-30 glyceryl triisostearate (monomer of)
- Peg-95 hexatriacontanyl ether (monomer of)
- PEG-25 beta-SITOSTERYL ETHER (monomer of)
- PEG-30 beta-SITOSTERYL ETHER (monomer of)
- Peg-4 alpha-linolenamide (monomer of)
- Peg/ppg-240/60 copolymer (monomer of)
- Peg-30 glyceryl stearate (monomer of)
- Peg-30 glyceryl linoleate (monomer of)
- Peg-4 glyceryl tristearate (monomer of)
- Peg/ppg-300/55 copolymer (monomer of)
- Peg-30 heneicosanyl ether (monomer of)
- Peg-30 stigmasteryl ether (monomer of)
- PEG-40 beta-SITOSTERYL ETHER (monomer of)
- Peg/ppg-35/9 copolymer (monomer of)
- Peg-32 glyceryl 1-stearate (monomer of)
- Peg-40 glyceryl isostearate (monomer of)
- Peg-40 dotriacontanyl ether (monomer of)
- Peg/ppg-4/30 copolymer (monomer of)
- Peg-4 glyceryl 1,3-distearate (monomer of)
- Peg-40 octacosanyl ether (monomer of)
- Peg-40 glyceryl triisostearate (monomer of)
- Polyethylene glycol 1100 (monomer of)
- Peg-40 glyceryl stearate (monomer of)
- Peg-40 tetracontanyl ether (monomer of)
- Peg-40 hexatriacontanyl ether (monomer of)
- Polyethylene glycol 250000 (monomer of)
- Peg-40 tetracosanyl ether (monomer of)
- Peg-40 triacontanyl ether (monomer of)
- Peg-40 stigmasteryl ether (monomer of)
- Polyethylene glycol 30000 (monomer of)
- PEG-5 beta-SITOSTERYL ETHER (monomer of)
- Peg-45 bis(N6-lysyl-succimide) (monomer of)
- Peg-40 tetratriacontanyl ether (monomer of)
- Polyethylene glycol 450 (monomer of)
- Peg-5 glyceryl stearate (monomer of)
- Peg-5 glyceryl isostearate (monomer of)
- Peg-45 bis(glycinyl-succimide) (monomer of)
- Polyethylene glycol 5000 (monomer of)
- Peg-5 glyceryl tristearate (monomer of)
- Peg-5 glyceryl triisostearate (monomer of)
- Peg-5 campesteryl ether (monomer of)
- Polyethylene glycol 650 (monomer of)
- Peg-5 stigmasteryl ether (monomer of)
- Peg-50 glyceryl triisostearate (monomer of)
- Peg-50 glyceryl isostearate (monomer of)
- Polyoxyl 40 Distearate (monomer of)
- Peg-7 caprylic/capric glycerides (monomer of)
- Peg-6 glyceryl isostearate (monomer of)
- Peg-6 glyceryl caprate (monomer of)
- Sodium laureth-12 carboxylate (monomer of)
- Peg-9 dimethicone (400 cst) (monomer of)
- Peg-60 glyceryl triisostearate (monomer of)
- Peg-60 glyceryl stearate (monomer of)
- Sodium laureth-7 carboxylate (monomer of)
- Peg-9 glyceryl isostearate (monomer of)
- Peg-7 glyceryl laurate (monomer of)
- Peg-95 hexacosanyl ether (monomer of)
- Sodium noneth-6 carboxylate (monomer of)
- Peg-95 dotriacontanyl ether (monomer of)
- PEG-90 Glyceryl Isostearate (monomer of)
- Peg-95 octatriacontanyl ether (monomer of)
- Sorbeth-12 hexacaprylate (monomer of)
- Peg-95 octacosanyl ether (monomer of)
- Peg-95 tetracontanyl ether (monomer of)
- Peg-95 tetratriacontanyl ether (monomer of)
- Sorbeth-12 hexalinoleate (monomer of)
- Peg-95 tetracosanyl ether (monomer of)
- Peg-95 triacontanyl ether (monomer of)
- Peg/ppg-25/30 copolymer (monomer of)
- Steareth-60 myristyl glycol (monomer of)
- Peg/ppg-18/4 copolymer (monomer of)
- Peg/ppg-60/11 glycerin (monomer of)
- Pegpleranib 5'-modification (monomer of)
- Succinyl mpeg 200 lysine (monomer of)
- Peg/ppg-5/30 copolymer (monomer of)
- Phenylalanine-peg 3400-proline (monomer of)
- Pentadeceth-6 phosphate (monomer of)
- Tetronic-701 tetrabenzoate (monomer of)
- Pentadeceth-9 phosphate (monomer of)
- Polyethylene glycol 1200 (monomer of)
- Pentadeceth-8 carboxylic acid (monomer of)
- Polyethylene glycol 14000 (monomer of)
- Polyethylene glycol 1300 (monomer of)
- Polyethylene glycol 500 (monomer of)
- Polyethylene glycol 1350 (monomer of)
- Polyethylene glycol 9000 (monomer of)
- Polyethylene glycol 35000 (monomer of)
- Sodium deceth-6 carboxylate (monomer of)
- Polyquaternium-10 (20000 mpa.S AT 2%) (monomer of)
- Sodium laureth-15 sulfonate (monomer of)
- Sodium laureth-3 carboxylate (monomer of)
- Sodium myreth-7 carboxylate (monomer of)
- Prolyl peg-230 cysteine linker (monomer of)
- Sodium trideceth-15 sulfonate (monomer of)
- Sodium laureth-8 carboxylate (monomer of)
- Sorbeth-12 hexacaprate (monomer of)
- Sodium myreth-15 sulfonate (monomer of)
- Sorbeth-12 hexapalmitate (monomer of)
- Sodium myreth-12 carboxylate (monomer of)
- Steareth-6 palmitoleate (monomer of)
- Sodium trideceth-12 carboxylate (monomer of)
- Succinyl mpeg 5000 lysine (monomer of)
- Sodium trideceth-4 carboxylate (monomer of)
- Threonine peg-80 proline (monomer of)
- Sodium trideceth-5 carboxylate (monomer of)
- Vitamin E Polyethylene Glycol Succinate (monomer of)
- Sorbeth-12 hexamyristate (monomer of)
- Sorbeth-12/oleate/dimer dilinoleate crosspolymer (monomer of)
- Sorbeth-12 hexastearate (monomer of)
Steardimonium hydroxypropyl PEG-7 dimethicone phosphate chloride (monomer of)
- Succinyl mpeg 20000 lysine (monomer of)
- Triceteth-5 phosphate (monomer of)
- Triceteth-4 phosphate (monomer of)
- Trideceth-9 phosphate (monomer of)
- Trilaureth-4 phosphate (monomer of)
- Tristeareth-4 phosphate (monomer of)
- Bempegaldesleukin pegylated lysine (monomer of)
Polyvinyl alcohol graft polyethylene glycol copolymer (3:1; 45000 MW) (monomer of)
- Diethylaminoethyl peg-5 myristate (monomer of)
- Avacincaptad pegol 5'-modification (monomer of)
- Cetyl hydroxyethylcellulose (550000 MW) (monomer of)
- Diethylaminoethyl peg-5 laurate (monomer of)
- Diethylaminoethyl peg-5 stearate (monomer of)
- Cetrimonium carboxydecyl peg-8 dimethicone (monomer of)
- Cysteine, damoctocog alfa pegol- (monomer of)
- Disodium 1-laureth-12 sulfosuccinate (monomer of)
- Dihydroxypropyl peg-10 stearammonium chloride (monomer of)
- Cetyl peg/ppg-10/1 dimethicone (hlb 3) (monomer of)
- Decyltetradeceth-200 isostearate (monomer of)
- Disodium 1-sec-myreth-12 sulfosuccinate (monomer of)
- Disodium 1-laureth sulfosuccinate (monomer of)
- Cysteinyl polymer, vasculotide- (monomer of)
- Diethylaminoethyl peg-5 caprylate (monomer of)
- Disodium 4-laureth sulfosuccinate (monomer of)
- Disodium 1-laureth-9 sulfosuccinate (monomer of)
- Diethylaminoethyl peg-5 caprate (monomer of)
- Disodium 1-myreth sulfosuccinate (monomer of)
- Disodium 4-laureth-9 sulfosuccinate (monomer of)
- Disodium 1-pentadeceth sulfosuccinate (monomer of)
- Diethylaminoethyl peg-5 linoleate (monomer of)
- Disodium 1-sec-laureth-12 sulfosuccinate (monomer of)
- Disodium 4-pentadeceth sulfosuccinate (monomer of)
- Disodium 1-sec-trideceth-12 sulfosuccinate (monomer of)
- Diethylaminoethyl peg-5 palmitate (monomer of)
- Disodium 1-trideceth sulfosuccinate (monomer of)
- Glycinyl-succimide-peg-45-succinic acid (monomer of)
- Disodium 4-oleth-3 sulfosuccinate (monomer of)
- Dilinoleic acid/glycol copolymer (monomer of)
- Disodium 4-laureth-6 sulfosuccinate (monomer of)
- Hydroxyethyl cellulose (40 mpa.S AT 2%) (monomer of)
- Disodium 4-sec-myreth-12 sulfosuccinate (monomer of)
- Disodium 1-laureth-6 sulfosuccinate (monomer of)
- Disodium 4-sec-trideceth-12 sulfosuccinate (monomer of)
- Hydroxypropylcocoate peg-8 dimethicone (monomer of)
- Disodium ethylene dilauramide peg-15 disulfate (monomer of)
- Disodium 1-oleth-3 sulfosuccinate (monomer of)
- Disodium ethylene dimyristamide peg-15 disulfate (monomer of)
- M-Peg-20000 N-succinylamide-serine (monomer of)
- Emapticap pegol 5'-modification (monomer of)
- Disodium 4-laureth-12 sulfosuccinate (monomer of)
- Lauryl peg-8 dimethicone (300 cps) (monomer of)
- M-Peg-5000 N-glutarimide-alanine (monomer of)
- M-Peg-20000 N6-succinylamide-lysine (monomer of)
- Disodium 4-myreth sulfosuccinate (monomer of)
- Magnesium 4-laureth-3 sulfosuccinate (monomer of)
- Mecapegfilgrastim modified L-methionine (monomer of)
- Mpeg-450 glutarate N-hydroxysuccimide (monomer of)
- Disodium 4-sec-laureth-12 sulfosuccinate (monomer of)
- Mpeg-110 glutarate N-hydroxysuccimide (monomer of)
- Methoxy peg 5000 succinimidyl carboxymethyl ester (monomer of)
- N6-Lysyl-succimide-peg-45-succinic acid (monomer of)
- Disodium 4-trideceth sulfosuccinate (monomer of)
- Mpeg-4 N-succinylamide-methionine (monomer of)
- Monosteareth-4 phosphate sodium (monomer of)
- Peg-10 glyceryl 1,2-diisostearate (monomer of)
- Lonapegsomatropin mpeg 40000 lysine (monomer of)
- Mpeg-propionaldehyde (20000 MW) (monomer of)
- Mpeg-220 glutarate N-hydroxysuccimide (monomer of)
PEG-14 1-((methylphenyl)ethyl)phenyl ether ammonium sulfate (monomer of)
- M-Peg-20000 N6-glutarimide-lysine (monomer of)
- N-(Mpeg-30000-propyl)-methionine (monomer of)
- Mpeg-900 glutarate N-hydroxysuccimide (monomer of)
- Peg-15 glyceryl 1,3-diisostearate (monomer of)
- M-Peg-5000 N6-glutarimide-lysine (monomer of)
- N-Pegylated branched glutamic acid (MW 43000) (monomer of)
- Mpeg-propionaldehyde (30000 MW) (monomer of)
- Peg-18 glyceryl oleate/caprylate (monomer of)
- Mpeg-680 glutarate N-hydroxysuccimide (monomer of)
- Peg-10 glyceryl 1,3-diisostearate (monomer of)
- Mpeg20000-carbamoyl-4-hydroxybutanamide-lysine (monomer of)
- Peg-18 glyceryl oleate/myristate (monomer of)
- Mpeg-propionaldehyde (10000 MW) (monomer of)
Peg-150/decyl alcohol/smdi copolymer (1350 MPA.S at 3%) (monomer of)
- N-(Mpeg-40000-propyl)-methionine (monomer of)
- Peg-30 glyceryl 1,3-diisostearate (monomer of)
- N-(Mpeg-20000-propyl)-methionine (monomer of)
- Peg-20 glyceryl 1,2-diisostearate (monomer of)
- N6-Lysyl-succimide-peg-45-succimide glycine (monomer of)
- Peg-5 caprylic/capric glycerides (monomer of)
- Navepegritide lysine modification (monomer of)
- Peg-7 trimethylolpropane cetyl ether (monomer of)
- Peg-18 glyceryl oleate/linoleate (monomer of)
- Peg-7 trimethylolpropane caprylic ether (monomer of)
- Olaptesed pegol sodium 5'-modification (monomer of)
- Peg-7 trimethylolpropane myristyl ether (monomer of)
- Peg-18 glyceryl oleate/palmitate (monomer of)
- Potassium dimethicone peg-7 phosphate (monomer of)
- Palsucibep pegol mpeg linker (30000 MW) (monomer of)
- Peg/ppg-32/10 aminopropyl methyl ether (monomer of)
- Peg-20 glyceryl 1,3-diisostearate (monomer of)
- Ppg-14 deceth-60 hexyl dicarbamate (monomer of)
- Peg-15 glyceryl 1,2-diisostearate (monomer of)
- Potassium trideceth-6 phosphate (monomer of)
- Peg-60 glyceryl 1,2-diisostearate (monomer of)
- Ppg-14 myreth-60 hexyl dicarbamate (monomer of)
- Peg-18 glyceryl oleate/stearate (monomer of)
- Ppg-14 capryleth-60 hexyl dicarbamate (monomer of)
- Peg-7 trimethylolpropane capryl ether (monomer of)
- Sodium pentadeceth-15 sulfonate (monomer of)
- Peg-30 glyceryl 1,2-diisostearate (monomer of)
- Ppg-14 laureth-60 hexyl dicarbamate (monomer of)
- Peg-7 trimethylolpropane lauryl ether (monomer of)
- Ppg-14 oleth-60 hexyl dicarbamate (monomer of)
- Peg-60 glyceryl 1,3-diisostearate (monomer of)
- Sodium pentadeceth-7 carboxylate (monomer of)
- Peg-7 trimethylolpropane oleyl ether (monomer of)
- Peg-7 trimethylolpropane linoleyl ether (monomer of)
- Pegnivacogin guanosine 5' modification (monomer of)
- Peg-7 trimethylolpropane stearyl ether (monomer of)
- Ppg-14 linoleth-60 hexyl dicarbamate (monomer of)
- Pegpleranib sodium 5'-modification (monomer of)
- Ppg-14 steareth-60 hexyl dicarbamate (monomer of)
- Potassium dimethicone peg-7 panthenyl phosphate (4 panthenol) (monomer of)
- Ppg-14 ceteth-60 hexyl dicarbamate (monomer of)
- Sodium carboxydecyl peg-8 dimethicone (monomer of)
- Sodium pentadeceth-12 carboxylate (monomer of)
- Carbomer interpolymer type C (allyl sucrose crosslinked) (monomer of)
- 1,3-Mpeg(2X-20KD)carboxamide-glycerol-maleimide-cysteinyl (monomer of)
- Disodium ethylene lauramide, myristamide peg-15 disulfate (monomer of)
- 4-(Mpeg-20000-carbonylaminoethoxyiminoethyl)phenylalanine (monomer of)
- N-(N-Mpeg-450-N-oxomethyl-mpeg-450-glycinyl)methionine (monomer of)
- Tyrosine peg-900 (adrenomedullin pegol modification) (monomer of)
- Hydroxyethyl fragment (degree OF polymerization 1.7) (monomer of)
- Dimethicone peg-7 panthenyl phosphate (12 panthenol) (monomer of)
- N6-(N-Mpeg-450-N-oxomethyl-mpeg-450-glycinyl)lysine (monomer of)
- M-Peg-2000-carbamoyl-ethyl-para-acetoxime-phenylalanine (monomer of)
- Methoxy polyethylene glycol propyl serine (20000 MW) (monomer of)
Steardimonium hydroxypropyl panthenyl PEG-7 dimethicone phosphate chloride (monomer of)
- Y-Branched-M-peg-40000-hexylamine-2'methyl-guanosine (monomer of)
- Pegaptanib, pegylated 2'-deoxy-2'-fluorouridine 5'-monophosphate- (monomer of)
- 1,2-Dimyristoyl-sn-glycero-3-carboxaminopropylpolyethylene glycol 2000 methyl ether (monomer of)
- 1,2-Distearoyl-rac-glycero-3-methylpolyoxyethylene (2000 MW) (monomer of)
- 2,3-Bis(methylpolyoxyethylene-oxy)-1-((3-oxopropylaminocarbonyl)oxy)-propane, (40000 MW) (monomer of)
- alpha-SUCCINIMIDYLOXY CARBONYL-omega-METHOXY, POLYOXYETHYLENE MW 12000 (monomer of)
- N-((Methyl-polyethylene glycol 24000)-2(RS)-2-methyl-propyl)methionine (monomer of)
- Pegaptanib sodium, pegylated 2'-deoxy-2'-fluorouridine 5'-monophosphate- (monomer of)
- N-(Carbonyl-methoxypolyethylene glycol 2000)-distearoyl-glycerophosphoethanolamine (monomer of)
- Antihemophilic factor, pegylated (MW 20000) human sequence recombinant pegylated lysine (monomer of)
Poly (2,2,4,4-tetramethyl-20-(oxiranylmethyl)-7-oxa-3,20-diazadispiro(5.1.11.2)heneicosan-21-one) (monomer of)
- N-(Carbonyl-methoxypolyethylene glycol 2000)-1,2-distearoyl-SN-glycero-3-phosphoethanolamine (monomer of)
- Bis-mono-methoxy branched polyethylene glycol, N-hydroxysuccinimide ester (40KD) (monomer of)
- N-(Carbonyl-methoxypolyethylenglycol 2000)-1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine sodium (monomer of)
- N-(Carbonyl-methoxypolyethylenglycol 2000)-1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine (monomer of)
- Sodium N-(carbonyl-methoxypolyethylene glycol 2000)-1,2-distearoyl-SN-glycero-3-phosphoethanolamine (monomer of)
- Sodium N-(carbonyl-methoxypolyethylene glycol-2000)-distearoyl-rac-glycerophosphoethanolamine (monomer of)
- Sodium N-(carbonyl-methoxypolyethylene glycol 5000)-1,2-distearoyl-SN-glycero-3-phosphoethanolamine (monomer of)
- S-(1-(3-((3-Hydroxypropyl)amino)-3-oxopropyl)-2,5-dioxo-3-pyrrolidinyl)cysteinyl methoxy peg-30 ether (monomer of)
- Monosodium N-(methoxypolyethylene glycol 5000 carbamoyl)-1,2-dipalmitoyl-SN-glycero-3-phosphatidylethanolamine (monomer of)
Ethylene oxide is used mainly as a chemical intermediate in the manufacture of ethylene glycol (antifreeze), textiles, detergents, polyurethane foam, solvents, medicine, adhesives, and other products.
Relatively small amounts of ethylene oxide are used as a fumigant, as a sterilant for food (spices) and cosmetics, and in hospital sterilization of surgical equipment and plastic devices that cannot be sterilized by steam.
- Monomers
- Surface active agents
- Processing aids not otherwise specified
- Finishing agents
- Functional fluids (closed systems)
- Intermediate
- Intermediates
- Intermediates
- Intermediate
- Functional fluids (closed systems)
- Surface active agents
- Finishing agents
- Processing aids not otherwise specified
Cosmetics product ingredient: Ethylene oxide (Oxirane)
Also known as: oxirane; dihydrooxirene; dimethylene oxide; epoxyethane; oxane
Source: Ethylene oxide is a highly reactive gas with a slightly sweet odor. Ethylene oxide is used primarily in the synthesis of other chemicals for use in perfumes and other cosmetics. It is also used in small amounts to sterilize food and cosmetics.
Potential health impacts: People are mainly exposed to ethylene oxide from inhaling ethylene oxide vapors. Studies of female rats given ethylene oxide orally found higher rates of stomach cancer. Studies of mice exposed to ethylene oxide by inhalation showed an increase in lung, uterine, and breast tumors. Many large occupational studies show that workers exposed to ethylene oxide have higher rates of breast, lymphatic, and hematopoietic (blood) cancers. The International Agency for Research on Cancer (IARC) categorizes ethylene oxide as a human carcinogen. California Proposition 65 lists ethylene oxide as both a carcinogen and a developmental toxin.
Product count: 97
Information on 66 consumer products that contain Ethylene oxide in the following categories is provided:
• Auto Products
• Commercial / Institutional
• Home Maintenance
• Inside the Home
• Personal Care
• Pet Care
CHEMICAL PROFILE: Ethylene oxide. End-use Pattern for Ethylene Oxide in 1987
Table: End-use Pattern for Ethylene Oxide in 1987
2019: 5,000,000,000 - <10,000,000,000 lb
2018: 5,000,000,000 - <10,000,000,000 lb
2017: 5,000,000,000 - <10,000,000,000 lb
2016: 5,000,000,000 - <10,000,000,000 lb
- Soap, Cleaning Compound, and Toilet Preparation Manufacturing
- Petrochemical Manufacturing
- All Other Chemical Product and Preparation Manufacturing
- Oil and Gas Drilling, Extraction, and Support activities
- Construction
- Paper Manufacturing
- Pesticide, Fertilizer, and Other Agricultural Chemical Manufacturing
- All Other Basic Organic Chemical Manufacturing
- Wholesale and Retail Trade
- Miscellaneous Manufacturing
- Asphalt Paving, Roofing, and Coating Materials Manufacturing



H315 (40.2%): Causes skin irritation [Warning Skin corrosion/irritation]
H317 (40.2%): May cause an allergic skin reaction [Warning Sensitization, Skin]
H318 (40.2%): Causes serious eye damage [Danger Serious eye damage/eye irritation]
H411 (59%): Toxic to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard]
H412 (40.2%): Harmful to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard]
P261, P264, P264+P265, P272, P273, P280, P302+P352, P305+P354+P338, P317, P321, P332+P317, P333+P317, P362+P364, P391, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 117 reports by companies from 3 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Reported as not meeting GHS hazard criteria per 1 of 117 reports by companies. For more detailed information, please visit ECHA C&L website.
There are 2 notifications provided by 116 of 117 reports by companies with hazard statement code(s).
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Skin Irrit. 2 (40.2%)
Skin Sens. 1B (40.2%)
Eye Dam. 1 (40.2%)
Aquatic Chronic 2 (59%)
Aquatic Chronic 3 (40.2%)
Flam. Gas 1 (> 99.9%)
Flam. Gas 1, Chem. Unst. Gas A (17.7%)
Press. Gas (Liq.) (85.8%)
Acute Tox. 3 (19.8%)
Acute Tox. 4 (62.1%)
Skin Corr. 1 (19.5%)
Skin Irrit. 2 (80.5%)
Eye Dam. 1 (19.5%)
Eye Irrit. 2 (80.5%)
Acute Tox. 3 (99.3%)
STOT SE 3 (> 99.9%)
STOT SE 3 (19.7%)
Muta. 1B (> 99.9%)
Carc. 1B (> 99.9%)
Repr. 1B (18.9%)
STOT RE 1 (78.8%)

- Chemical: Ethylene Oxide
- Threshold: 5000 [lb]
- Note: Ethylene Oxide in quantities at or above above 5000lb presents a potential for a catastrophic event as a toxic or reactive highly hazardous chemical.
· TOXIC; may be fatal if inhaled or absorbed through skin. Some may cause severe skin burns and eye damage.
· Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite.
· Fire will produce irritating, corrosive and/or toxic gases.
· Runoff from fire control or dilution water may cause environmental contamination.
· Flammable; may be ignited by heat, sparks or flames.
· May form explosive mixtures with air. Ethylene oxide (UN1040) may react explosively even in the absence of air.
· Those substances designated with a (P) may polymerize explosively when heated or involved in a fire.
· Vapors from liquefied gas are initially heavier than air and spread along ground.
· Vapors may travel to source of ignition and flash back.
· Some of these materials may react violently with water.
· Cylinders exposed to fire may vent and release toxic and flammable gas through pressure relief devices.
· Containers may explode when heated.
· Ruptured cylinders may rocket.
· Runoff may create fire or explosion hazard.
NR=Not recommended
AEGLs Status: Final
Warning: Ethylene oxide is corrosive to moist tissues. Caution is advised.
Signs and Symptoms of Acute Ethylene Oxide Exposure: Signs and symptoms of acute exposure to ethylene oxide may be severe, and include dyspnea (shortness of breath), cough, pulmonary edema, pneumonia, and respiratory failure. Lethargy, headache, dizziness, twitching, convulsions, paralysis, and coma may be observed. Cardiac arrhythmias and cardiovascular collapse may also occur. Gastrointestinal effects of acute exposure may include nausea, vomiting, and abdominal pain. Ethylene oxide may severely irritate or burn mucous membranes and moist skin. Eye contact may result in conjunctivitis (red, inflamed eyes) and erosion of the cornea.
Emergency Life-Support Procedures: Acute exposure to ethylene oxide may require decontamination and life support for the victims. Emergency personnel should wear protective clothing appropriate to the type and degree of contamination. Air-purifying or supplied-air respiratory equipment should also be worn, as necessary. Rescue vehicles should carry supplies such as plastic sheeting and disposable plastic bags to assist in preventing spread of contamination.
Inhalation Exposure:
1. Move victims to fresh air. Emergency personnel should avoid self-exposure to ethylene oxide.
2. Evaluate vital signs including pulse and respiratory rate, and note any trauma. If no pulse is detected, provide CPR. If not breathing, provide artificial respiration. If breathing is labored, administer oxygen or other respiratory support.
3. Obtain authorization and/or further instructions from the local hospital for administration of an antidote or performance of other invasive procedures.
4. Transport to a health care facility.
Dermal/Eye Exposure:
1. Remove victims from exposure. Emergency personnel should avoid self- exposure to ethylene oxide.
2. Evaluate vital signs including pulse and respiratory rate, and note any trauma. If no pulse is detected, provide CPR. If not breathing, provide artificial respiration. If breathing is labored, administer oxygen or other respiratory support.
3. Remove contaminated clothing as soon as possible.
4. If eye exposure has occurred, eyes must be IMMEDIATELY flushed with lukewarm water for AT LEAST 15 minutes.
5. If liquid is spilled on the skin, allow ethylene oxide to vaporize before washing THOROUGHLY with soap and water.
6. Obtain authorization and/or further instructions from the local hospital for administration of an antidote or performance of other invasive procedures.
7. Transport to a health care facility.
Ingestion Exposure:
1. Evaluate vital signs including pulse and respiratory rate, and note any trauma. If no pulse is detected, provide CPR. If not breathing, provide artificial respiration. If breathing is labored, administer oxygen or other respiratory support.
2. Obtain authorization and/or further instructions from the local hospital for administration of an antidote or performance of other invasive procedures.
3. Give the victims water or milk: children up 1 year old, 125 mL (4 oz or 1/2 cup); children 1 to 12 years old 200 mL (6 oz or 3/4 cup); adults, 250 mL (8 oz or 1 cup). Water or milk should be given only if victims are conscious and alert.
4. Activated charcoal may be administered if victims are conscious and alert. Use 15 to 30 g (1/2 to 1 oz) for children, 50 to 100 g (1-3/4 to 3-1/2 oz) for adults, with 125 to 250 mL (1/2 to 1 cup) of water.
5. Ethylene oxide generally acts as its own cathartic; however, if deemed necessary, excretion may be promoted by administering a saline cathartic or sorbitol to conscious and alert victims. Children require 15 to 30 g (1/2 to 1 oz) of cathartic; 50 to 100 g (1-3/4 to 3-1/2 oz) is recommended for adults.
6. Transport to a health care facility. (EPA, 1998)
General First Aid:
· Call 911 or emergency medical service.
· Ensure that medical personnel are aware of the material(s) involved, take precautions to protect themselves and avoid contamination.
· Move victim to fresh air if it can be done safely.
· Administer oxygen if breathing is difficult.
· If victim is not breathing:
-- DO NOT perform mouth-to-mouth resuscitation; the victim may have ingestedor inhaled the substance.
-- If equipped and pulse detected, wash face and mouth, then give artificial respiration using a proper respiratory medical device (bag-valve mask, pocket mask equipped with a one-way valve or other device).
-- If no pulse detected or no respiratory medical device available, provide continuouscompressions. Conduct a pulse check every two minutes or monitor for any signs of spontaneous respirations.
· Remove and isolate contaminated clothing and shoes.
· For minor skin contact, avoid spreading material on unaffected skin.
· In case of contact with substance, remove immediately by flushing skin or eyes with running water for at least 20 minutes.
· For severe burns, immediate medical attention is required.
· Effects of exposure (inhalation, ingestion, or skin contact) to substance may be delayed.
· Keep victim calm and warm.
· Keep victim under observation.
· For further assistance, contact your local Poison Control Center.
· Note: Basic Life Support (BLS) and Advanced Life Support (ALS) should be done by trained professionals.
Specific First Aid:
· In case of contact with liquefied gas, only medical personnel should attempt thawing frosted parts.
· In case of burns, immediately cool affected skin for as long as possible with cold water. Do not remove clothing if adhering to skin.
In Canada, an Emergency Response Assistance Plan (ERAP) may be required for this product. Please consult the shipping paper and/or the "ERAP" section.
(See general first aid procedures)
Eye: Irrigate immediately - If this chemical contacts the eyes, immediately wash (irrigate) the eyes with large amounts of water, occasionally lifting the lower and upper lids. Get medical attention immediately.
Skin: Water flush immediately - If this chemical contacts the skin, immediately flush the contaminated skin with water. If this chemical penetrates the clothing, immediately remove the clothing and flush the skin with water. Get medical attention promptly.
Breathing: Respiratory support
Swallow: Medical attention immediately (liquid)
Move container from fire area if you can do so without risk. Stay away from ends of tanks. Fight fire from maximum distance. For massive fire in cargo area, use unmanned hose holder or monitor nozzles; if this is impossible, withdraw from area and let fire burn. Withdraw immediately in case of rising sound from venting safety device or any discoloration of tank due to fire. Isolate for 1 mile in all directions if tank car or truck is involved in fire. Keep unnecessary people away; isolate hazard area and deny entry. Stay upwind; keep out of low areas. Wear positive pressure breathing apparatus and full protective clothing. Evacuate area endangered by gas.
Extinguish with alcohol foam, carbon dioxide, dry chemical or water spray, fog, or foam. Let burn unless leak can be stopped immediately. (EPA, 1998)
· CALL 911. Then call emergency response telephone number on shipping paper. If shipping paper not available or no answer, refer to appropriate telephone number listed on the inside back cover.
· Keep unauthorized personnel away.
· Stay upwind, uphill and/or upstream.
· Many gases are heavier than air and will spread along the ground and collect in low or confined areas (sewers, basements, tanks, etc.).
· Ventilate closed spaces before entering, but only if properly trained and equipped.
· ELIMINATE all ignition sources (no smoking, flares, sparks or flames) from immediate area.
· All equipment used when handling the product must be grounded.
· Do not touch or walk through spilled material.
· Stop leak if you can do it without risk.
· Do not direct water at spill or source of leak.
· Use water spray to reduce vapors or divert vapor cloud drift. Avoid allowing water runoff to contact spilled material.
· FOR CHLOROSILANES, use alcohol-resistant foam to reduce vapors.
· If possible, turn leaking containers so that gas escapes rather than liquid.
· Prevent entry into waterways, sewers, basements or confined areas.
· Isolate area until gas has dispersed.
Excerpt from ERG Guide 119 [Gases - Toxic - Flammable; polymerization hazard]:
IMMEDIATE PRECAUTIONARY MEASURE: Isolate spill or leak area for at least 100 meters (330 feet) in all directions.
SPILL: See ERG Tables 1 and 3 - Initial Isolation and Protective Action Distances on the UN/NA 1040 datasheet.
FIRE: If tank, rail tank car or highway tank is involved in a fire, ISOLATE for 1600 meters (1 mile) in all directions; also, consider initial evacuation for 1600 meters (1 mile) in all directions. (ERG, 2024)
Immediate precautionary measure
· Isolate spill or leak area for at least 100 meters (330 feet) in all directions.
Spill
· For highlighted materials: see Table 1 - Initial Isolation and Protective Action Distances.
· For non-highlighted materials: increase the immediate precautionary measure distance, in the downwind direction, as necessary.
Fire
· If tank, rail tank car or highway tank is involved in a fire, ISOLATE for 1600 meters (1 mile) in all directions; also, consider initial evacuation for 1600 meters (1 mile) in all directions.
Small spill:
- ISOLATE in all directions: 30 m (100 ft)
Large spill:
- ISOLATE in all Directions:
-- Rail tank car: 200 m (600 ft)
-- Rail tank car: 200 m (600 ft)
-- Rail tank car: 200 m (600 ft)
-- Highway tank truck or trailer: 100 m (300 ft)
-- Highway tank truck or trailer: 100 m (300 ft)
-- Highway tank truck or trailer: 100 m (300 ft)
-- Multiple small cylinders or single ton cylinder: 30 m (100 ft)
-- Multiple small cylinders or single ton cylinder: 30 m (100 ft)
-- Multiple small cylinders or single ton cylinder: 30 m (100 ft)
Small spill:
- PROTECT people from downwind during DAY time: 0.1 km (0.1 mi)
- PROTECT people from downwind during NIGHT time: 0.2 km (0.2 mi)
Large spill:
- PROTECT people from downwind during DAY time:
-- Rail tank car:
- - - Low wind (< 6 mph (<10 km/h)): 1.5 km (1.0 mi)
- - - Moderate wind (6-12 mph (10-20 km/h)): 0.8 km (0.5 mi)
- - - High wind (> 12 mph (>20 km/h)): 0.7 km (0.4 mi)
-- Rail tank car:
- - - Low wind (< 6 mph (<10 km/h)): 1.5 km (1.0 mi)
- - - Moderate wind (6-12 mph (10-20 km/h)): 0.8 km (0.5 mi)
- - - High wind (> 12 mph (>20 km/h)): 0.7 km (0.4 mi)
-- Rail tank car:
- - - Low wind (< 6 mph (<10 km/h)): 1.5 km (1.0 mi)
- - - Moderate wind (6-12 mph (10-20 km/h)): 0.8 km (0.5 mi)
- - - High wind (> 12 mph (>20 km/h)): 0.7 km (0.4 mi)
-- Highway tank truck or trailer:
- - - Low wind (< 6 mph (<10 km/h)): 0.9 km (0.6 mi)
- - - Moderate wind (6-12 mph (10-20 km/h)): 0.5 km (0.3 mi)
- - - High wind (> 12 mph (>20 km/h)): 0.4 km (0.3 mi)
-- Highway tank truck or trailer:
- - - Low wind (< 6 mph (<10 km/h)): 0.9 km (0.6 mi)
- - - Moderate wind (6-12 mph (10-20 km/h)): 0.5 km (0.3 mi)
- - - High wind (> 12 mph (>20 km/h)): 0.4 km (0.3 mi)
-- Highway tank truck or trailer:
- - - Low wind (< 6 mph (<10 km/h)): 0.9 km (0.6 mi)
- - - Moderate wind (6-12 mph (10-20 km/h)): 0.5 km (0.3 mi)
- - - High wind (> 12 mph (>20 km/h)): 0.4 km (0.3 mi)
-- Multiple small cylinders or single ton cylinder:
- - - Low wind (< 6 mph (<10 km/h)): 0.4 km (0.3 mi)
- - - Moderate wind (6-12 mph (10-20 km/h)): 0.2 km (0.1 mi)
- - - High wind (> 12 mph (>20 km/h)): 0.1 km (0.1 mi)
-- Multiple small cylinders or single ton cylinder:
- - - Low wind (< 6 mph (<10 km/h)): 0.4 km (0.3 mi)
- - - Moderate wind (6-12 mph (10-20 km/h)): 0.2 km (0.1 mi)
- - - High wind (> 12 mph (>20 km/h)): 0.1 km (0.1 mi)
-- Multiple small cylinders or single ton cylinder:
- - - Low wind (< 6 mph (<10 km/h)): 0.4 km (0.3 mi)
- - - Moderate wind (6-12 mph (10-20 km/h)): 0.2 km (0.1 mi)
- - - High wind (> 12 mph (>20 km/h)): 0.1 km (0.1 mi)
Large spill:
- PROTECT people from downwind during NIGHT time:
-- Rail tank car:
- - - Low wind (< 6 mph (<10 km/h)): 3.0 km (1.8 mi)
- - - Moderate wind (6-12 mph (10-20 km/h)): 1.4 km (0.9 mi)
- - - High wind (> 12 mph (>20 km/h)): 0.8 km (0.5 mi)
-- Rail tank car:
- - - Low wind (< 6 mph (<10 km/h)): 3.0 km (1.8 mi)
- - - Moderate wind (6-12 mph (10-20 km/h)): 1.4 km (0.9 mi)
- - - High wind (> 12 mph (>20 km/h)): 0.8 km (0.5 mi)
-- Rail tank car:
- - - Low wind (< 6 mph (<10 km/h)): 3.0 km (1.8 mi)
- - - Moderate wind (6-12 mph (10-20 km/h)): 1.4 km (0.9 mi)
- - - High wind (> 12 mph (>20 km/h)): 0.8 km (0.5 mi)
-- Highway tank truck or trailer:
- - - Low wind (< 6 mph (<10 km/h)): 2.0 km (1.3 mi)
- - - Moderate wind (6-12 mph (10-20 km/h)): 0.7 km (0.4 mi)
- - - High wind (> 12 mph (>20 km/h)): 0.4 km (0.3 mi)
-- Highway tank truck or trailer:
- - - Low wind (< 6 mph (<10 km/h)): 2.0 km (1.3 mi)
- - - Moderate wind (6-12 mph (10-20 km/h)): 0.7 km (0.4 mi)
- - - High wind (> 12 mph (>20 km/h)): 0.4 km (0.3 mi)
-- Highway tank truck or trailer:
- - - Low wind (< 6 mph (<10 km/h)): 2.0 km (1.3 mi)
- - - Moderate wind (6-12 mph (10-20 km/h)): 0.7 km (0.4 mi)
- - - High wind (> 12 mph (>20 km/h)): 0.4 km (0.3 mi)
-- Multiple small cylinders or single ton cylinder:
- - - Low wind (< 6 mph (<10 km/h)): 0.8 km (0.5 mi)
- - - Moderate wind (6-12 mph (10-20 km/h)): 0.3 km (0.2 mi)
- - - High wind (> 12 mph (>20 km/h)): 0.2 km (0.1 mi)
-- Multiple small cylinders or single ton cylinder:
- - - Low wind (< 6 mph (<10 km/h)): 0.8 km (0.5 mi)
- - - Moderate wind (6-12 mph (10-20 km/h)): 0.3 km (0.2 mi)
- - - High wind (> 12 mph (>20 km/h)): 0.2 km (0.1 mi)
-- Multiple small cylinders or single ton cylinder:
- - - Low wind (< 6 mph (<10 km/h)): 0.8 km (0.5 mi)
- - - Moderate wind (6-12 mph (10-20 km/h)): 0.3 km (0.2 mi)
- - - High wind (> 12 mph (>20 km/h)): 0.2 km (0.1 mi)
Excerpt from ERG Guide 119 [Gases - Toxic - Flammable; polymerization hazard]:
ELIMINATE all ignition sources (no smoking, flares, sparks or flames) from immediate area. All equipment used when handling the product must be grounded. Do not touch or walk through spilled material. Stop leak if you can do it without risk. Do not direct water at spill or source of leak. Use water spray to reduce vapors or divert vapor cloud drift. Avoid allowing water runoff to contact spilled material. FOR CHLOROSILANES, use alcohol-resistant foam to reduce vapors. If possible, turn leaking containers so that gas escapes rather than liquid. Prevent entry into waterways, sewers, basements or confined areas. Isolate area until gas has dispersed. (ERG, 2024)
· Wear positive pressure self-contained breathing apparatus (SCBA).
· Wear chemical protective clothing that is specifically recommended by the manufacturer when there is NO RISK OF FIRE.
· Structural firefighters' protective clothing provides thermal protection but only limited chemical protection.
Biological Exposure Indices (BEI) [ACGIH] - N-(2-hydroxyethyl)valine (HEV) hemoglobin adducts = 5000 pmol HEV/g globin. See urine BEI and further details. [TLVs and BEIs]
TIH (Toxic Inhalation Hazard) - Term used to describe gases and volatile liquids that are toxic when inhaled. Some are TIH materials themselves, e.g., chlorine, and some release TIH gases when spilled in water, e.g., chlorosilanes. [ERG 2016].
800.0 [ppm]
Excerpts from Documentation for IDLHs: Reports of effects in humans include: nasal irritation after exposures to 12,500 ppm for 10 sec. Acute studies in animals have shown: death after exposure > 8000 ppm for 10 min.; no apparent injuries after exposure to 4000 ppm for 30 min., 2000 ppm for 60 min., or 500 ppm for 1 hr.
Ca [800 ppm]
See: 75218
· DO NOT EXTINGUISH A LEAKING GAS FIRE UNLESS LEAK CAN BE STOPPED.
Small Fire
· Dry chemical, CO2, water spray or alcohol-resistant foam.
Large Fire
· Water spray, fog or alcohol-resistant foam.
· FOR CHLOROSILANES, DO NOT USE WATER; use alcohol-resistant foam.
· If it can be done safely, move undamaged containers away from the area around the fire.
· Damaged cylinders should be handled only by specialists.
Fire Involving Tanks
· Fight fire from maximum distance or use unmanned master stream devices or monitor nozzles.
· Cool containers with flooding quantities of water until well after fire is out.
· Do not direct water at source of leak or safety devices; icing may occur.
· Withdraw immediately in case of rising sound from venting safety devices or discoloration of tank.
· ALWAYS stay away from tanks in direct contact with flames.
ERPG-1: Not appropriate - one hour exposure limit: 1 = mild transient health effects or objectionable odor [AIHA]
ERPG-2: 50 ppm - one hour exposure limit: 2 = impaired ability to take protective action [AIHA]
ERPG-3: 500 ppm - one hour exposure limit: 3 = life threatening health effects [AIHA]
Excerpt from NIOSH Pocket Guide for Ethylene oxide:
Skin: PREVENT SKIN CONTACT (LIQUID) - If this chemical is in liquid form, wear appropriate personal protective clothing to prevent skin contact.
Eyes: PREVENT EYE CONTACT (LIQUID) - If this chemical is in liquid form, wear appropriate eye protection to prevent eye contact.
Wash skin: WHEN CONTAMINATED (LIQUID) - If this chemical is in liquid form, the worker should immediately wash the skin when it becomes contaminated.
Remove: WHEN WET (FLAMMABLE) - Work clothing that becomes wet should be immediately removed due to its flammability hazard (i.e., for liquids with a flash point <100 °F).
Change: No recommendation is made specifying the need for the worker to change clothing after the workshift.
Provide: QUICK DRENCH (LIQUID) - Facilities for quickly drenching the body should be provided (when this chemical is in liquid form) within the immediate work area for emergency use where there is a possibility of exposure. [Note: It is intended that these facilities provide a sufficient quantity or flow of water to quickly remove the substance from any body areas likely to be exposed. The actual determination of what constitutes an adequate quick drench facility depends on the specific circumstances. In certain instances, a deluge shower should be readily available, whereas in others, the availability of water from a sink or hose could be considered adequate.] (NIOSH, 2024)
(See personal protection and sanitation codes)
Skin: Prevent skin contact (liquid)
Eyes: Prevent eye contact (liquid)
Wash skin: When contaminated (liquid)
Remove: When wet (flammable)
Change: No recommendation
Provide: Quick drench (liquid)
(See OSHA respirator requirements for selected chemicals)
NIOSH
Up to 5 ppm:
(APF = 50) Any air-purifying, full-facepiece respirator (gas mask) with a chin-style, front- or back-mounted canister providing protection against the compound of concern†
(APF = 50) Any self-contained breathing apparatus with a full facepiece
(APF = 50) Any supplied-air respirator with a full facepiece
Emergency or planned entry into unknown concentrations or IDLH conditions:
(APF = 10,000) Any self-contained breathing apparatus that has a full facepiece and is operated in a pressure-demand or other positive-pressure mode
(APF = 10,000) Any supplied-air respirator that has a full facepiece and is operated in a pressure-demand or other positive-pressure mode in combination with an auxiliary self-contained positive-pressure breathing apparatus
Escape:
(APF = 50) Any air-purifying, full-facepiece respirator (gas mask) with a chin-style, front- or back-mounted canister providing protection against the compound of concern†
Any appropriate escape-type, self-contained breathing apparatus
Epoxides
Polymerizable Compounds
Highly Flammable
Explosive
Polymerizable
If ... THERE IS NO FIRE, go directly to the Table of Initial Isolation and Protective Action Distances /(see table below)/ ... to obtain initial isolation and protective action distances. IF THERE IS A FIRE, or IF A FIRE IS INVOLVED, go directly to the appropriate guide /(see guide(s) below)/ and use the evacuation information shown under PUBLIC SAFETY.
Table: Table of Isolation and Protective Action Distances for Ethylene oxide
Hazard Traits - Carcinogenicity; Dermatotoxicity; Developmental Toxicity; Genotoxicity; Hazard Trait Under Review; Hematotoxicity; Neurotoxicity; Reproductive Toxicity; Respiratory Toxicity
Authoritative List - ATSDR Neurotoxicants; CA TACs; EC Annex VI CMRs - Cat. 1B; IARC Carcinogens - 1; IRIS Carcinogens - Carcin.; NTP RoC - known; OEHHA RELs; Prop 65
Report - regardless of intended function of ingredient in the product
Status: Active Update: 31-10-2012 https://echa.europa.eu/registration-dossier/-/registered-dossier/1668
Status: Cease Manufacture Update: 01-05-2018 https://echa.europa.eu/registration-dossier/-/registered-dossier/24308
Status: Active Update: 12-01-2011 https://echa.europa.eu/registration-dossier/-/registered-dossier/6191
Status: Active Update: 08-11-2012 https://echa.europa.eu/registration-dossier/-/registered-dossier/8062
Status: Cease Manufacture Update: 27-07-2010 https://echa.europa.eu/registration-dossier/-/registered-dossier/1229
Status: Active Update: 18-05-2023 https://echa.europa.eu/registration-dossier/-/registered-dossier/15813
Volume Sup 7: Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42, 1987; 440 pages; ISBN 92-832-1411-0 (out of print)
Volume 60: (1994) Some Industrial Chemicals
Volume 97: (2008) 1,3-Butadiene, Ethylene Oxide and Vinyl Halides (Vinyl Fluoride, Vinyl Chloride and Vinyl Bromide)
Volume 100F: (2012) Chemical Agents and Related Occupations
 Chemical Not Tested in Species/Sex
Chemical Not Tested in Species/Sex Chemical Not Tested in Species/Sex
Chemical Not Tested in Species/Sex Clear Evidence
Clear Evidence Clear Evidence
Clear EvidenceImmune
Reproductive
Neurotoxin - Sensorimotor
Occupational hepatotoxin - Secondary hepatotoxins: the potential for toxic effect in the occupational setting is based on cases of poisoning by human ingestion or animal experimentation.
Reproductive Toxin - A chemical that is toxic to the reproductive system, including defects in the progeny and injury to male or female reproductive function. Reproductive toxicity includes developmental effects. See Guidelines for Reproductive Toxicity Risk Assessment.
Dermatotoxin - Skin burns.
Skin Sensitizer - An agent that can induce an allergic reaction in the skin.
Asthma - Reversible bronchoconstriction (narrowing of bronchioles) initiated by the inhalation of irritating or allergenic agents.
Toxic Pneumonitis - Inflammation of the lungs induced by inhalation of metal fumes or toxic gases and vapors.
IARC Carcinogen - Class 1: International Agency for Research on Cancer classifies chemicals as established human carcinogens.
NTP Carcinogen - Known to be a human carcinogen.
ACGIH Carcinogen - Suspected Human.
Spontaneous abortions [Category: Reproduction and Development]
Cataract, chemical or radiation induced [Category: Chronic Poisoning]
Contact dermatitis, allergic [Category: Skin Disease]
Fumigants, acute toxic effect [Category: Acute Poisoning]
Pneumonitis, toxic [Category: Acute Poisoning]
Asthma, occupational [Category: Airway Disease]
Neuropathy, toxic [Category: Chronic Poisoning]
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=IAYPIBMASNFSPL-UHFFFAOYSA-N
- Agency for Toxic Substances and Disease Registry (ATSDR)LICENSEThe information provided using CDC Web site is only intended to be general summary information to the public. It is not intended to take the place of either the written law or regulations.https://www.cdc.gov/Other/disclaimer.html
- The National Institute for Occupational Safety and Health (NIOSH)LICENSEThe information provided using CDC Web site is only intended to be general summary information to the public. It is not intended to take the place of either the written law or regulations.https://www.cdc.gov/Other/disclaimer.htmlEthylene oxidehttps://www.cdc.gov/niosh/npg/npgd0275.htmlEthylene oxidehttps://www.cdc.gov/niosh-rtecs/KX256250.html
- EPA Air Toxics
- Haz-Map, Information on Hazardous Chemicals and Occupational DiseasesLICENSECopyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://haz-map.com/AboutEthylene oxidehttps://haz-map.com/Agents/21
- California Office of Environmental Health Hazard Assessment (OEHHA)
- CAMEO ChemicalsLICENSECAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data.https://cameochemicals.noaa.gov/help/reference/terms_and_conditions.htm?d_f=falseETHYLENE OXIDEhttps://cameochemicals.noaa.gov/chemical/694CAMEO Chemical Reactivity Classificationhttps://cameochemicals.noaa.gov/browse/react
- ChEBI
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/Ethylene Oxidehttps://www.wikidata.org/wiki/Q407473LOTUS Treehttps://lotus.naturalproducts.net/
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- Toxin and Toxin Target Database (T3DB)LICENSET3DB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (T3DB) and the original publication.http://www.t3db.ca/downloadsEthylene Oxidehttp://www.t3db.ca/toxins/T3D4207
- Australian Industrial Chemicals Introduction Scheme (AICIS)
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/Ethylene oxidehttps://commonchemistry.cas.org/detail?cas_rn=75-21-8
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DHS Chemical Facility Anti-Terrorism Standards (CFATS) Chemicals of Interest
- EPA Acute Exposure Guideline Levels (AEGLs)
- EPA Chemical Data Reporting (CDR)LICENSEThe U.S. Government retains a nonexclusive, royalty-free license to publish or reproduce these documents, or allow others to do so, for U.S. Government purposes. These documents may be freely distributed and used for non-commercial, scientific and educational purposes.https://www.epa.gov/web-policies-and-procedures/epa-disclaimers#copyright
- EPA Chemicals under the TSCAEPA TSCA Classificationhttps://www.epa.gov/tsca-inventory
- EPA DSSToxEthylene oxidehttps://comptox.epa.gov/dashboard/DTXSID0020600Oxiranyl radicalhttps://comptox.epa.gov/dashboard/DTXSID30185475CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- EPA Integrated Risk Information System (IRIS)
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeEthylene oxidehttps://chem.echa.europa.eu/100.000.773Oxirane (EC: 617-762-0)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/7739Ethylene oxide (EC: 200-849-9)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/26125
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Hazardous Substances Data Bank (HSDB)ETHYLENE OXIDEhttps://pubchem.ncbi.nlm.nih.gov/source/hsdb/170
- ILO-WHO International Chemical Safety Cards (ICSCs)
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/
- NJDOH RTK Hazardous Substance Listethylene oxidehttp://nj.gov/health/eoh/rtkweb/documents/fs/0882.pdf
- Occupational Safety and Health Administration (OSHA)LICENSEMaterials created by the federal government are generally part of the public domain and may be used, reproduced and distributed without permission. Therefore, content on this website which is in the public domain may be used without the prior permission of the U.S. Department of Labor (DOL). Warning: Some content - including both images and text - may be the copyrighted property of others and used by the DOL under a license.https://www.dol.gov/general/aboutdol/copyrightETHYLENE OXIDEhttps://www.osha.gov/chemicaldata/575
- Risk Assessment Information System (RAIS)LICENSEThis work has been sponsored by the U.S. Department of Energy (DOE), Office of Environmental Management, Oak Ridge Operations (ORO) Office through a joint collaboration between United Cleanup Oak Ridge LLC (UCOR), Oak Ridge National Laboratory (ORNL), and The University of Tennessee, Ecology and Evolutionary Biology, The Institute for Environmental Modeling (TIEM). All rights reserved.https://rais.ornl.gov/Ethylene Oxidehttps://rais.ornl.gov/cgi-bin/tools/TOX_search
- California Safe Cosmetics Program (CSCP) Product DatabaseEthylene oxide (Oxirane)https://cscpsearch.cdph.ca.gov/search/detailresult/311
- Consumer Product Information Database (CPID)LICENSECopyright (c) 2024 DeLima Associates. All rights reserved. Unless otherwise indicated, all materials from CPID are copyrighted by DeLima Associates. No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://www.whatsinproducts.com/contents/view/1/6Consumer Products Category Classificationhttps://www.whatsinproducts.com/
- Emergency Response Guidebook (ERG)Ethylene oxidehttps://pubchem.ncbi.nlm.nih.gov/erg/
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jspEthylene Oxidehttps://ctdbase.org/detail.go?type=chem&acc=D005027
- Crystallography Open Database (COD)LICENSEAll data in the COD and the database itself are dedicated to the public domain and licensed under the CC0 License. Users of the data should acknowledge the original authors of the structural data.https://creativecommons.org/publicdomain/zero/1.0/
- The Cambridge Structural Database
- EPA Chemical and Products Database (CPDat)EPA CPDat Classificationhttps://www.epa.gov/chemical-research/chemical-and-products-database-cpdat
- Joint FAO/WHO Expert Committee on Food Additives (JECFA)LICENSEPermission from WHO is not required for the use of WHO materials issued under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Intergovernmental Organization (CC BY-NC-SA 3.0 IGO) licence.https://www.who.int/about/policies/publishing/copyright
- EPA Regional Screening Levels for Chemical Contaminants at Superfund SitesEthylene Oxidehttps://epa-prgs.ornl.gov/cgi-bin/chemicals/csl_search
- EU Pesticides Database
- Hazardous Chemical Information System (HCIS), Safe Work Australia
- NITE-CMCEthylene oxide - FY2006 (New/original classication)https://www.chem-info.nite.go.jp/chem/english/ghs/06-imcg-0012e.htmlEthylene oxide - FY2011 (Revised classification)https://www.chem-info.nite.go.jp/chem/english/ghs/11-mhlw-2005e.html
- Regulation (EC) No 1272/2008 of the European Parliament and of the CouncilLICENSEThe copyright for the editorial content of this source, the summaries of EU legislation and the consolidated texts, which is owned by the EU, is licensed under the Creative Commons Attribution 4.0 International licence.https://eur-lex.europa.eu/content/legal-notice/legal-notice.htmlethylene oxide; oxiranehttps://eur-lex.europa.eu/eli/reg/2008/1272/oj
- FDA Substances Added to FoodLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- SpectraBaseETHYLENE OXIDEhttps://spectrabase.com/spectrum/41mUh7wvbJoEthylene oxidehttps://spectrabase.com/spectrum/3dtADfczQ0ETHYLENEOXIDE;OXIRANEhttps://spectrabase.com/spectrum/8wesNyPr27sETHYLENE OXIDEhttps://spectrabase.com/spectrum/4PD2G3yprgOEthylene oxidehttps://spectrabase.com/spectrum/7XrFG6UeUyG
- MassBank Europe
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingEthylene oxidehttp://www.hmdb.ca/metabolites/HMDB0255987HMDB0255987_nmr_one_2613https://hmdb.ca/metabolites/HMDB0255987#spectra
- International Agency for Research on Cancer (IARC)LICENSEMaterials made available by IARC/WHO enjoy copyright protection under the Berne Convention for the Protection of Literature and Artistic Works, under other international conventions, and under national laws on copyright and neighbouring rights. IARC exercises copyright over its Materials to make sure that they are used in accordance with the Agency's principles. All rights are reserved.https://publications.iarc.fr/Terms-Of-UseEthylene oxidehttps://monographs.iarc.who.int/list-of-classificationsIARC Classificationhttps://www.iarc.fr/
- NTP Technical ReportsEthylene Oxidehttps://ntp.niehs.nih.gov/data/tr
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlTherapeutic category of drugs in Japanhttp://www.genome.jp/kegg-bin/get_htext?br08301.kegRisk category of Japanese OTC drugshttp://www.genome.jp/kegg-bin/get_htext?br08312.kegClassification of Japanese OTC drugshttp://www.genome.jp/kegg-bin/get_htext?br08313.kegAnimal drugs in Japanhttp://www.genome.jp/kegg-bin/get_htext?br08331.keg
- Natural Product Activity and Species Source (NPASS)
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawEthylene oxidehttp://www.nist.gov/srd/nist1a.cfm
- Metabolomics Workbench
- NIOSH Manual of Analytical MethodsLICENSEThe information provided using CDC Web site is only intended to be general summary information to the public. It is not intended to take the place of either the written law or regulations.https://www.cdc.gov/Other/disclaimer.html
- NMRShiftDB
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/Ethylene OxideNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- Pistoia Alliance Chemical Safety LibraryEthylene oxide + Trimethylaminehttps://safescience.cas.org/
- Springer Nature
- SpringerMaterials
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidataethylene oxidehttps://www.wikidata.org/wiki/Q407473
- Wikipediaethylene oxidehttps://en.wikipedia.org/wiki/Ethylene_oxide
- Wiley
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlEthylene Oxidehttps://www.ncbi.nlm.nih.gov/mesh/68005027Disinfectantshttps://www.ncbi.nlm.nih.gov/mesh/68004202
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403029532https://pubchem.ncbi.nlm.nih.gov/substance/403029532
- NCBI







