Sodium Benzoate
- sodium benzoate
- 532-32-1
- Sobenate
- Antimol
- Benzoic acid, sodium salt
- Create:2005-03-27
- Modify:2025-01-18
 Benzoic Acid (has active moiety); Sodium benzoate; sodium phenylacetate (component of) ... View More ...
Benzoic Acid (has active moiety); Sodium benzoate; sodium phenylacetate (component of) ... View More ...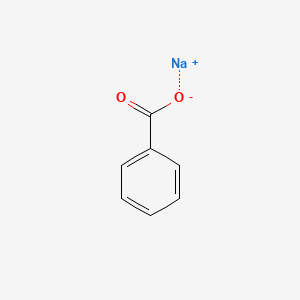
C7H5NaO2
C6H5COONa
- Benzoate, Sodium
- Sodium Benzoate
- sodium benzoate
- 532-32-1
- Sobenate
- Antimol
- Benzoic acid, sodium salt
- Benzoic acid sodium salt
- Benzoate sodium
- Benzoate of soda
- Benzoate, sodium
- sodium;benzoate
- Natrium benzoicum
- FEMA No. 3025
- Fuminaru
- Benzoan sodny
- Caswell No. 746
- Microcare sb
- PUROX S
- FEMA Number 3025
- CCRIS 3921
- HSDB 696
- Benzoesaeure (na-salz)
- UNII-OJ245FE5EU
- EINECS 208-534-8
- OJ245FE5EU
- benzoic acid sodium
- MFCD00012463
- EPA Pesticide Chemical Code 009103
- INS NO.211
- DTXSID1020140
- E211
- AI3-07835
- INS-211
- DTXCID90140
- Sodiumbenzoate
- E-211
- CHEBI:113455
- EC 208-534-8
- AMMONUL COMPONENT SODIUM BENZOATE
- UCEPHAN COMPONENT SODIUM BENZOATE
- SODIUM BENZOATE (II)
- SODIUM BENZOATE [II]
- SODIUM BENZOATE (MART.)
- SODIUM BENZOATE [MART.]
- SODIUM BENZOATE (USP-RS)
- SODIUM BENZOATE [USP-RS]
- Benzoan sodny [Czech]
- SODIUM BENZOATE (EP MONOGRAPH)
- SODIUM BENZOATE [EP MONOGRAPH]
- Benzoesaeure (na-salz) [German]
- s panax mist
- BzONa
- Sodium benzoate [USAN:JAN:NF]
- monosodium benzoate
- Sodium Benzoate,(S)
- Sodium benzoate (TN)
- s panax mist for refill
- s panax all in one mist
- SCHEMBL823
- CHEMBL1356
- SODIUM BENZOATE [MI]
- Sodium benzoate (JP18/NF)
- SODIUM BENZOATE [FCC]
- SODIUM BENZOATE [JAN]
- SODIUM BENZOATE [FHFI]
- SODIUM BENZOATE [HSDB]
- SODIUM BENZOATE [USAN]
- SODIUM BENZOATE [VANDF]
- SODIUM BENZOATE [WHO-DD]
- Benzoic acid, sodium salt (1:1)
- HY-Y1316
- Tox21_300125
- SODIUM BENZOATE [ORANGE BOOK]
- AKOS003053000
- AKOS015890021
- CCG-266169
- NCGC00254072-01
- CAS-532-32-1
- DA-57965
- SODIUM BENZOATE COMPONENT OF AMMONUL
- SODIUM BENZOATE COMPONENT OF UCEPHAN
- CS-0017788
- NS00074364
- S0593
- D02277
- A829462
- Q423971
- J-519752
- Flavor and Extract Manufacturers' Association No. 3025
Benzoic Acid (has active moiety)
- Sodium benzoate; sodium phenylacetate (component of)
- Arsenic trioxide; aspartame; atropa belladonna; benzalkonium chloride; chelidonium majus; formaldehyde; lycopodium clavatum spore; methylene chloride; methylparaben; phosphoric acid; phytolacca americana root; propylparaben; sodium benzoate; sodium citrate; titanium dioxide; trifolium pratense flower; zanthoxylum americanum bark; zinc oxide (component of)
- Arsenic trioxide; aspartame; benzalkonium chloride; beta vulgaris; bisphenol A; chelidonium majus; cobalt; copper; formaldehyde; glyphosate; iodine; lycopodium clavatum spore; methylene chloride; methylparaben; petroselinum crispum; peumus boldus leaf; phosphoric acid; propylparaben; selenium; sodium benzoate; sodium citrate; taraxacum officinale; titanium dioxide; zinc; zinc oxide (component of)
- Arsenic trioxide; aspartame; benzalkonium chloride; bisphenol A; chelidonium majus whole; cobalt; copper; formaldehyde solution; glyphosate; iodine; lycopodium clavatum spore; methylene chloride; methylparaben; milk thistle; petroselinum crispum whole; peumus boldus leaf; phosphoric acid; propylparaben; selenium; sodium benzoate; sodium citrate, unspecified form; taraxacum officinale; titanium dioxide; zinc; zinc oxide (component of)
- Acetic acid; anhydrous trisodium citrate; arsenic trioxide; aspartame; benzalkonium chloride; benzoic acid; benzyl alcohol; boric acid; chlorine; cholesterol; corticotropin; cortisone acetate; estradiol; estrone; eugenol; formaldehyde solution; isopropyl palmitate; lactic acid, DL-; lead; lycopodium clavatum spore; methylene chloride; methylparaben; petrolatum; phenylbutazone; phosphoric acid; phosphorus; potassium sorbate; progesterone; propylparaben; resorcinol; salicylic acid; sodium benzoate; sorbitol; squalene; stearyl alcohol; strychnos nux-vomica seed; titanium dioxide; xylitol; zinc oxide (component of)
Reported uses (ppm):
Table: Reported uses (ppm): (Flavor and Extract Manufacturers' Association, 1994)
 Green circle - The chemical has been verified to be of low concern
Green circle - The chemical has been verified to be of low concern- Pigments
- Other
- Abrasives
- Finishing agents
- Preservative
- Paint additives and coating additives not described by other categories
- Processing aids, not otherwise listed
- Surface active agents
- Antioxidant
- Lubricants and lubricant additives
- Other (specify)
- Laboratory chemicals
- Adhesives and sealant chemicals
- Functional fluids (closed systems)
- Emulsifier
- Not Known or Reasonably Ascertainable
- Cleaning agent
- Agricultural chemicals (non-pesticidal)
- Preservative
- Corrosion inhibitor
- Pigments
- Not Known or Reasonably Ascertainable
- Functional fluids (closed systems)
- Emulsifier
- Adhesives and sealant chemicals
- Laboratory chemicals
- Lubricants and lubricant additives
- Other (specify)
- Adhesion/cohesion promoter
- Antioxidant
Information on 874 consumer products that contain Sodium benzoate in the following categories is provided:
• Auto Products
• Commercial / Institutional
• Hobby/Craft
• Home Maintenance
• Inside the Home
• Personal Care
• Pesticides
• Pet Care
2019: 20,000,000 lb - <100,000,000 lb
2018: 20,000,000 lb - <100,000,000 lb
2017: 20,000,000 lb - <100,000,000 lb
2016: 20,000,000 lb - <100,000,000 lb
- Other (requires additional information)
- All Other Basic Organic Chemical Manufacturing
- Paint and Coating Manufacturing
- All Other Basic Inorganic Chemical Manufacturing
- Miscellaneous Manufacturing
- Wholesale and Retail Trade
- Textiles, apparel, and leather manufacturing
- Petroleum Lubricating Oil and Grease Manufacturing
- Food, beverage, and tobacco product manufacturing
- Mining (except Oil and Gas) and support activities
- Not Known or Reasonably Ascertainable
- Plastics Material and Resin Manufacturing
- Soap, Cleaning Compound, and Toilet Preparation Manufacturing
- Construction
- Oil and Gas Drilling, Extraction, and Support activities
- All Other Chemical Product and Preparation Manufacturing
- Pharmaceutical and Medicine Manufacturing
- Fabricated Metal Product Manufacturing
- Plastics Product Manufacturing

P264+P265, P280, P305+P351+P338, and P337+P317
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 2902 reports by companies from 12 notifications to the ECHA C&L Inventory.
Reported as not meeting GHS hazard criteria per 1827 of 2902 reports by companies. For more detailed information, please visit ECHA C&L website.
There are 10 notifications provided by 1075 of 2902 reports by companies with hazard statement code(s).
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Chemical: Benzoic acid, sodium Salt

IMAP assessments - Benzoic acid, sodium salt: Environment tier I assessment
IMAP assessments - Benzoic acid, sodium salt: Human health tier I assessment
Nephrotoxin - The chemical is potentially toxic to the kidneys in the occupational setting.
ACGIH Carcinogen - Not Suspected.
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=WXMKPNITSTVMEF-UHFFFAOYSA-M
- Australian Industrial Chemicals Introduction Scheme (AICIS)Benzoic acid, sodium salt (1:1)https://services.industrialchemicals.gov.au/search-assessments/Benzoic acid, sodium salt (1:1)https://services.industrialchemicals.gov.au/search-inventory/
- ChemIDplusSodium benzoate [USAN:JAN:NF]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000532321ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA Chemical Data Reporting (CDR)LICENSEThe U.S. Government retains a nonexclusive, royalty-free license to publish or reproduce these documents, or allow others to do so, for U.S. Government purposes. These documents may be freely distributed and used for non-commercial, scientific and educational purposes.https://www.epa.gov/web-policies-and-procedures/epa-disclaimers#copyrightBenzoic acid, sodium salt (1:1)https://www.epa.gov/chemical-data-reporting
- EPA Chemicals under the TSCABenzoic acid, sodium salt (1:1)https://www.epa.gov/chemicals-under-tscaEPA TSCA Classificationhttps://www.epa.gov/tsca-inventory
- EPA DSSToxSodium benzoatehttps://comptox.epa.gov/dashboard/DTXSID1020140CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeSodium benzoatehttps://chem.echa.europa.eu/100.007.760Sodium benzoate (EC: 208-534-8)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/125419
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Hazardous Substances Data Bank (HSDB)SODIUM BENZOATEhttps://pubchem.ncbi.nlm.nih.gov/source/hsdb/696
- ILO-WHO International Chemical Safety Cards (ICSCs)
- International Fragrance Association (IFRA)LICENSE(c) The International Fragrance Association, 2007-2021. All rights reserved.https://ifrafragrance.org/links/copyright
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/
- ChEBISodium benzoatehttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:113455
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licenceSODIUM BENZOATEhttps://platform.opentargets.org/drug/CHEMBL1356
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jspSodium Benzoatehttps://ctdbase.org/detail.go?type=chem&acc=D020160
- Haz-Map, Information on Hazardous Chemicals and Occupational DiseasesLICENSECopyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://haz-map.com/AboutSodium benzoatehttps://haz-map.com/Agents/7079
- Therapeutic Target Database (TTD)Sodium benzoatehttps://idrblab.net/ttd/data/drug/details/D0SC6I
- Consumer Product Information Database (CPID)LICENSECopyright (c) 2024 DeLima Associates. All rights reserved. Unless otherwise indicated, all materials from CPID are copyrighted by DeLima Associates. No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://www.whatsinproducts.com/contents/view/1/6Consumer Products Category Classificationhttps://www.whatsinproducts.com/
- Cosmetic Ingredient Review (CIR)
- EPA Chemical and Products Database (CPDat)EPA CPDat Classificationhttps://www.epa.gov/chemical-research/chemical-and-products-database-cpdat
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/Sodium BenzoateNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- DailyMed
- Drugs@FDALICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingSODIUM BENZOATEhttps://www.accessdata.fda.gov/scripts/cder/daf/
- EU Food Improvement AgentsSODIUM BENZOATEhttp://data.europa.eu/eli/reg/2012/231/2024-04-23
- EPA Safer ChoiceBenzoic acid, sodium Salthttps://www.epa.gov/saferchoice/safer-ingredientsEPA Safer Chemical Ingredients Classificationhttps://www.epa.gov/saferchoice
- FDA Orange BookLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Joint FAO/WHO Expert Committee on Food Additives (JECFA)LICENSEPermission from WHO is not required for the use of WHO materials issued under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Intergovernmental Organization (CC BY-NC-SA 3.0 IGO) licence.https://www.who.int/about/policies/publishing/copyright
- EU Clinical Trials Register
- NITE-CMCSodium benzoate; Benzoic acid, sodium salt - FY2022 (New/original classication)https://www.chem-info.nite.go.jp/chem/english/ghs/22-jniosh-0045e.html
- FDA Substances Added to FoodLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Flavor and Extract Manufacturers Association (FEMA)SODIUM BENZOATEhttps://www.femaflavor.org/flavor-library/sodium-benzoate
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingSodium benzoatehttp://www.hmdb.ca/metabolites/HMDB0303720
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlTherapeutic category of drugs in Japanhttp://www.genome.jp/kegg-bin/get_htext?br08301.kegAnatomical Therapeutic Chemical (ATC) classificationhttp://www.genome.jp/kegg-bin/get_htext?br08303.kegDrugs listed in the Japanese Pharmacopoeiahttp://www.genome.jp/kegg-bin/get_htext?br08311.kegRisk category of Japanese OTC drugshttp://www.genome.jp/kegg-bin/get_htext?br08312.keg
- Metabolomics Workbench
- National Drug Code (NDC) DirectoryLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Natural Product Activity and Species Source (NPASS)Sodium Benzoatehttps://bidd.group/NPASS/compound.php?compoundID=NPC318107
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- NIPH Clinical Trials Search of Japan
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.htmlsodium benzoatehttps://rxnav.nlm.nih.gov/id/rxnorm/56455
- PharmGKBLICENSEPharmGKB data are subject to the Creative Commons Attribution-ShareALike 4.0 license (https://creativecommons.org/licenses/by-sa/4.0/).https://www.pharmgkb.org/page/policiessodium benzoatehttps://www.pharmgkb.org/chemical/PA451374
- SpectraBaseBENZOIC ACID, SODIUM SALThttps://spectrabase.com/spectrum/FJw9rqDaAPnSODIUM BENZOATEhttps://spectrabase.com/spectrum/HxQ9LZXFI6SSodium benzoatehttps://spectrabase.com/spectrum/4pzo7YYRtsjSodium benzoatehttps://spectrabase.com/spectrum/INV4XDKrDaqBENZOIC ACID, SODIUM SALThttps://spectrabase.com/spectrum/1v2Ve27rEMmBENZOIC_ACID;SODIUM_SALThttps://spectrabase.com/spectrum/LMpqR81Y4eosodium benzoatehttps://spectrabase.com/spectrum/9ycClF6LfPMbenzoic acid, sodium salthttps://spectrabase.com/spectrum/GGWxHZA5Hj4SODIUM-BENZOATEhttps://spectrabase.com/spectrum/KGvzqVDhmfESODIUMBENZOATEhttps://spectrabase.com/spectrum/DP9tswk1aB2Sodium benzoatehttps://spectrabase.com/spectrum/CWYcx4XLxzHSodium benzoatehttps://spectrabase.com/spectrum/AtiForuVzZS
- Springer Nature
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- WHO Anatomical Therapeutic Chemical (ATC) ClassificationLICENSEUse of all or parts of the material requires reference to the WHO Collaborating Centre for Drug Statistics Methodology. Copying and distribution for commercial purposes is not allowed. Changing or manipulating the material is not allowed.https://www.whocc.no/copyright_disclaimer/Sodium benzoatehttps://www.whocc.no/atc_ddd_index/?code=A16AX11
- Wikidatasodium benzoatehttps://www.wikidata.org/wiki/Q423971
- Wikipediasodium benzoatehttps://en.wikipedia.org/wiki/Sodium_benzoate
- Wiley
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlSodium Benzoatehttps://www.ncbi.nlm.nih.gov/mesh/68020160Antifungal Agentshttps://www.ncbi.nlm.nih.gov/mesh/68000935Food Preservativeshttps://www.ncbi.nlm.nih.gov/mesh/68005520
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403029568https://pubchem.ncbi.nlm.nih.gov/substance/403029568

 CID 5360545 (Sodium)
CID 5360545 (Sodium)