Potassium Sorbate
- POTASSIUM SORBATE
- 24634-61-5
- Sorbistat potassium
- Sorbistat-K
- Potassium (E,E)-sorbate
- Create:2008-02-05
- Modify:2025-01-18
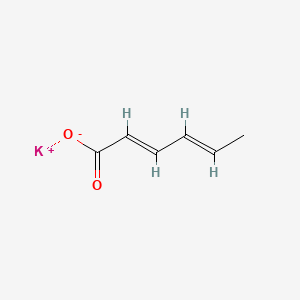
- Acid, Hexadienoic
- Acid, Propenylacrylic
- Acid, Sorbic
- Hexadienoic Acid
- Potassium Sorbate
- Propenylacrylic Acid
- Sodium Sorbate
- Sorbate, Potassium
- Sorbate, Sodium
- Sorbic Acid
- POTASSIUM SORBATE
- 24634-61-5
- Sorbistat potassium
- Sorbistat-K
- Potassium (E,E)-sorbate
- Sorbic acid potassium salt
- 590-00-1
- potassium (2E,4E)-hexa-2,4-dienoate
- Potassium 2,4-hexadienoate
- Sorbic acid, potassium salt
- BB Powder
- Sorbistat-potassium
- FEMA No. 2921
- Sorbistat k
- Potassium sorbate (E)
- Caswell No. 701C
- Potassium (E,E)-2,4-hexadienoate
- Potassium (E,E)-hexa-2,4-dienoate
- CCRIS 1894
- HSDB 1230
- Ins no.202
- Potassium (e,e')-sorbate
- UNII-1VPU26JZZ4
- EINECS 246-376-1
- Ins-202
- 1VPU26JZZ4
- 2,4-Hexadienoic acid, potassium salt
- potassium hexa-2,4-dienoate
- Potassium sorbate (e 202)
- EPA Pesticide Chemical Code 075902
- Potassium 2,4-hexadienoate, (E,E)-
- potassium;(2E,4E)-hexa-2,4-dienoate
- 2,4-Hexadienoic acid, potassium salt, (2E,4E)-
- CHEBI:77868
- AI3-26043
- E 202
- Potassium sorbate [NF]
- MFCD00016546
- 2,4-Hexadienoic acid, potassium salt, (E,E)-
- Sorbic acid, potassium salt, (E,E)-
- potassium trans,trans-sorbate
- 2,4-Hexadienoic acid, potassium salt (1:1), (2E,4E)-
- DTXSID7027835
- E-202
- 2,4-Hexadienoic acid potassium salt, (E,E)-
- 2,4-Hexadienoic acid, (E,E)-, potassium salt
- EC 246-376-1
- potassium trans,trans-2,4-hexadienoate
- Potassium sorbate (NF)
- POTASSIUM SORBATE (II)
- POTASSIUM SORBATE [II]
- 2,4-HEXADIENOIC ACID, (E,E')-, POTASSIUM SALT
- 2,4-Hexadienoic acid potassium salt
- POTASSIUM SORBATE (MART.)
- POTASSIUM SORBATE [MART.]
- POTASSIUM SORBATE (USP-RS)
- POTASSIUM SORBATE [USP-RS]
- Potassium Sorbate [USAN]
- POTASSIUM SORBATE (EP IMPURITY)
- POTASSIUM SORBATE [EP IMPURITY]
- POTASSIUM SORBATE (EP MONOGRAPH)
- POTASSIUM SORBATE [EP MONOGRAPH]
- Sorbic acid (potassium)
- trans-trans-Sorbic acid potassium
- Sorbate, Potassium
- 2,4-Hexadienoic acid, potassium salt (1:1)
- Potassium (E,E')-sorbate; Potassium sorbate
- SCHEMBL3640
- Potassium sorbate (Standard)
- DTXCID207835
- HY-N0626AR
- POTASSIUM SORBATE [FCC]
- CHEMBL2106930
- POTASSIUM SORBATE [FHFI]
- HY-N0626A
- POTASSIUM SORBATE [VANDF]
- POTASSIUM SORBATE [WHO-DD]
- Tox21_202757
- AKOS015915488
- 2,4-Hexadienoic acid, (E,E')-, potassium salt; 2,4-Hexadienoic acid, potassium salt
- SORBIC ACID POTASSIUM SALT [MI]
- NCGC00260304-01
- CAS-24634-61-5
- CS-0102519
- NS00094865
- P1954
- S0057
- D02411
- G73516
- Q410744
- J-015607
- J-524028
- trans-trans-Sorbic acid potassium 100 microg/mL in Water
67.0548 100
41.0383 89.52
65.0381 59.66
45.033 29.34
39.0218 28.55
67.0547 100
95.0491 97.24
57.0331 46.68
43.0163 25.51
113.0607 24.31
67.056587 100
65.040916 61.24
95.049278 39.91
71.930107 25.69
71.932510 18.81
Reported uses (ppm):
Table: Reported uses (ppm): (Flavor and Extract Manufacturers' Association, 1994)
 Green circle - The chemical has been verified to be of low concern
Green circle - The chemical has been verified to be of low concern- Processing aids, not otherwise listed
- Other (specify)
- Not Known or Reasonably Ascertainable
- Stabilizing agent
- Not Known or Reasonably Ascertainable
- Other (specify)
- Processing aids, not otherwise listed
- Preservative
Information on 39 consumer products that contain Potassium Sorbate [USAN] in the following categories is provided:
• Inside the Home
• Personal Care
• Pesticides
• Pet Care
Information on 241 consumer products that contain Potassium sorbate in the following categories is provided:
• Commercial / Institutional
• Inside the Home
• Personal Care
• Pesticides
• Pet Care
2019: 1,000,000 - <10,000,000 lb
2018: 1,000,000 - <10,000,000 lb
2017: 1,000,000 - <10,000,000 lb
2016: 1,000,000 - <10,000,000 lb
2019: 212,981 lb
2018: 341,981 lb
2017: 171,974 lb
2016: 126,000 lb
- Textiles, apparel, and leather manufacturing
- Food, beverage, and tobacco product manufacturing
- Agriculture, Forestry, Fishing and Hunting
- All Other Basic Organic Chemical Manufacturing
- Not Known or Reasonably Ascertainable

H315 (63.4%): Causes skin irritation [Warning Skin corrosion/irritation]
H319 (94.2%): Causes serious eye irritation [Warning Serious eye damage/eye irritation]
H335 (33.2%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation]
P261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 1698 reports by companies from 19 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Reported as not meeting GHS hazard criteria per 90 of 1698 reports by companies. For more detailed information, please visit ECHA C&L website.
There are 18 notifications provided by 1608 of 1698 reports by companies with hazard statement code(s).
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Skin Irrit. 2 (63.4%)
Eye Irrit. 2 (94.2%)
STOT SE 3 (33.2%)
Skin Irrit. 2 (47.9%)
Eye Irrit. 2 (56.8%)
STOT SE 3 (10.8%)
Chemical: Potassium (E,E)-sorbate

Chemical: Potassium sorbate

IMAP assessments - 2,4-Hexadienoic acid, potassium salt, (E,E)-: Human health tier I assessment
IMAP assessments - 2,4-Hexadienoic acid, potassium salt, (E,E)-: Environment tier I assessment
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=CHHHXKFHOYLYRE-STWYSWDKSA-M
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=ZABWAOACTAXLLP-STWYSWDKSA-M
- Australian Industrial Chemicals Introduction Scheme (AICIS)2,4-Hexadienoic acid, potassium salt (1:1), (2E,4E)-https://services.industrialchemicals.gov.au/search-assessments/2,4-Hexadienoic acid, potassium salt (1:1), (2E,4E)-https://services.industrialchemicals.gov.au/search-inventory/
- ChemIDplusPotassium Sorbate [USAN]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000590001Potassium sorbate [NF]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0024634615ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA Chemical Data Reporting (CDR)LICENSEThe U.S. Government retains a nonexclusive, royalty-free license to publish or reproduce these documents, or allow others to do so, for U.S. Government purposes. These documents may be freely distributed and used for non-commercial, scientific and educational purposes.https://www.epa.gov/web-policies-and-procedures/epa-disclaimers#copyright2,4-Hexadienoic acid, potassium salt (1:1), (2E,4E)-https://www.epa.gov/chemical-data-reporting
- EPA Chemicals under the TSCA2,4-Hexadienoic acid, potassium salt (1:1)https://www.epa.gov/chemicals-under-tscaEPA TSCA Classificationhttps://www.epa.gov/tsca-inventory
- EPA DSSToxPotassium (E,E)-hexa-2,4-dienoatehttps://comptox.epa.gov/dashboard/DTXSID7027835CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticePotassium (E,E)-hexa-2,4-dienoatehttps://chem.echa.europa.eu/100.042.1452,4-Hexadienoic acid, potassium salthttps://echa.europa.eu/substance-information/-/substanceinfo/100.130.671potassium (E,E)-hexa-2,4-dienoatehttps://echa.europa.eu/substance-information/-/substanceinfo/100.205.816Potassium (E,E)-hexa-2,4-dienoate (EC: 246-376-1)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/226442,4-Hexadienoic acid, potassium salt (EC: 611-771-3)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/120852potassium (E,E)-hexa-2,4-dienoate (EC: 680-685-6)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/210764
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingPOTASSIUM SORBATEhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/1VPU26JZZ4
- Hazardous Substances Data Bank (HSDB)POTASSIUM SORBATEhttps://pubchem.ncbi.nlm.nih.gov/source/hsdb/1230
- International Fragrance Association (IFRA)LICENSE(c) The International Fragrance Association, 2007-2021. All rights reserved.https://ifrafragrance.org/links/copyright2,4-Hexadienoic acid, potassium salt (1:1), (2E,4E)-https://ifrafragrance.org/priorities/ingredients/ifra-transparency-list
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/
- EU Pesticides Database
- ChEBIPotassium sorbatehttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:77868
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.html
- Consumer Product Information Database (CPID)LICENSECopyright (c) 2024 DeLima Associates. All rights reserved. Unless otherwise indicated, all materials from CPID are copyrighted by DeLima Associates. No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://www.whatsinproducts.com/contents/view/1/6Potassium Sorbate [USAN]https://www.whatsinproducts.com/chemicals/view/1/917/000590-00-1Potassium sorbatehttps://www.whatsinproducts.com/chemicals/view/1/3545/024634-61-5Consumer Products Category Classificationhttps://www.whatsinproducts.com/
- Cosmetic Ingredient Review (CIR)
- EPA Chemical and Products Database (CPDat)EPA CPDat Classificationhttps://www.epa.gov/chemical-research/chemical-and-products-database-cpdat
- Haz-Map, Information on Hazardous Chemicals and Occupational DiseasesLICENSECopyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://haz-map.com/AboutPotassium sorbatehttps://haz-map.com/Agents/6820
- DailyMed
- EU Food Improvement AgentsPOTASSIUM SORBATEhttp://data.europa.eu/eli/reg/2012/231/2024-04-23
- EPA Safer ChoicePotassium (E,E)-sorbatehttps://www.epa.gov/saferchoice/safer-ingredientsEPA Safer Chemical Ingredients Classificationhttps://www.epa.gov/saferchoice
- Joint FAO/WHO Expert Committee on Food Additives (JECFA)LICENSEPermission from WHO is not required for the use of WHO materials issued under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Intergovernmental Organization (CC BY-NC-SA 3.0 IGO) licence.https://www.who.int/about/policies/publishing/copyright
- Hazardous Chemical Information System (HCIS), Safe Work Australiapotassium (E,E)-hexa-2,4-dienoatehttp://hcis.safeworkaustralia.gov.au/HazardousChemical/Details?chemicalID=5661
- Regulation (EC) No 1272/2008 of the European Parliament and of the CouncilLICENSEThe copyright for the editorial content of this source, the summaries of EU legislation and the consolidated texts, which is owned by the EU, is licensed under the Creative Commons Attribution 4.0 International licence.https://eur-lex.europa.eu/content/legal-notice/legal-notice.htmlpotassium (E,E)-hexa-2,4-dienoatehttps://eur-lex.europa.eu/eli/reg/2008/1272/oj
- FDA Substances Added to FoodLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Flavor and Extract Manufacturers Association (FEMA)POTASSIUM SORBATEhttps://www.femaflavor.org/flavor-library/potassium-sorbate
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingPotassium sorbatehttp://www.hmdb.ca/metabolites/HMDB0303718
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.html
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- National Drug Code (NDC) DirectoryLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.htmlpotassium sorbatehttps://rxnav.nlm.nih.gov/id/rxnorm/8606
- SpectraBasesorbic acid, potassium salthttps://spectrabase.com/spectrum/58hOpNnKQ0Ktrans,trans-2,4-Hexadienoic acid potassium salthttps://spectrabase.com/spectrum/AK1Gwr9q1Xpsorbic acid, potassium salthttps://spectrabase.com/spectrum/KgClNGdkrAUPotassium sorbatehttps://spectrabase.com/spectrum/79ZdNK10WtQPotassium sorbatehttps://spectrabase.com/spectrum/7TlaZdV3920POTASSIUM SORBATEhttps://spectrabase.com/spectrum/LKbOsigZIZePOTASSIUM SORBATEhttps://spectrabase.com/spectrum/CUjaX4O7h1VSORBIC ACID, POTASSIUM SALThttps://spectrabase.com/spectrum/SWNq6EaR4Ktrans,trans-2,4-Hexadienoic acid potassium salthttps://spectrabase.com/spectrum/9ov3RujpzWn
- Springer Nature
- Wikidatapotassium sorbatehttps://www.wikidata.org/wiki/Q410744
- WikipediaSodium sorbatehttps://en.wikipedia.org/wiki/Sodium_sorbatePotassium sorbatehttps://en.wikipedia.org/wiki/Potassium_sorbate
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlSorbic Acidhttps://www.ncbi.nlm.nih.gov/mesh/68013011Food Preservativeshttps://www.ncbi.nlm.nih.gov/mesh/68005520
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403029385https://pubchem.ncbi.nlm.nih.gov/substance/403029385SID 445976348https://pubchem.ncbi.nlm.nih.gov/substance/445976348
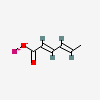

 CID 643460 (Sorbic Acid)
CID 643460 (Sorbic Acid) CID 5462222 (Potassium)
CID 5462222 (Potassium)

