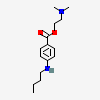
Tetracaine
- tetracaine
- 94-24-6
- Amethocaine
- 2-(Dimethylamino)ethyl 4-(butylamino)benzoate
- Pontocaine
- Create:2005-03-25
- Modify:2025-01-18
 Tetracaine Hydrochloride (has salt form); Lidocaine; Tetracaine (component of); Benzocaine; Lidocaine; Tetracaine (component of) ... View More ...
Tetracaine Hydrochloride (has salt form); Lidocaine; Tetracaine (component of); Benzocaine; Lidocaine; Tetracaine (component of) ... View More ...
- Amethocaine
- Ametop
- Dicaine
- Hydrochloride, Tetrracaine
- Pantocaine
- Pontocaine
- Tetracaine
- Tetracaine Monohydrochloride
- Tetrakain
- Tetrracaine Hydrochloride
- tetracaine
- 94-24-6
- Amethocaine
- 2-(Dimethylamino)ethyl 4-(butylamino)benzoate
- Pontocaine
- Dicaine
- Laudocaine
- Metraspray
- Tetracaina
- Tetrakain
- Anetain
- Uromucaesthin
- Contralgin
- Fissucain
- Intercain
- Meethobalm
- Mucaesthin
- Rexocaine
- Tetracainum
- Dicain
- Medihaler-Tetracaine
- Medicaine
- Niphanoid
- Dikain
- 2-(Dimethylamino)ethyl p-(butylamino)benzoate
- p-Butylaminobenzoyl-2-dimethylaminoethanol
- Benzoic acid, 4-(butylamino)-, 2-(dimethylamino)ethyl ester
- Diaethylaminoaethanol ester der p-butylaminobenzoesaeure
- Tetracainum [INN-Latin]
- Dimethylaminoethyl p-butyl-aminobenzoate
- Tetracaina [INN-Spanish]
- CHEBI:9468
- Amethocaine HCl
- EINECS 202-316-6
- MFCD00053787
- 4-(Butylamino)benzoic acid 2-(dimethylamino)ethyl ester
- p-(Butylamino)benzoic acid, 2-(dimethylamino)ethyl ester
- UNII-0619F35CGV
- BRN 2216051
- BENZOIC ACID, p-(BUTYLAMINO)-, 2-(DIMETHYLAMINO)ETHYL ESTER
- 2-Dimethylaminoethylester kyseliny p-butylaminobenzoove
- 0619F35CGV
- CHEMBL698
- Tetracaine [USP:INN:BAN]
- DTXSID1043883
- Tetracaine Base
- SYNERA COMPONENT TETRACAINE
- 4-14-00-01172 (Beilstein Handbook Reference)
- Tetrakain [Czech]
- p-(butylamino)benzoic acid beta-(dimethylamino)ethyl ester
- Tetracainum (INN-Latin)
- Tetracaina (INN-Spanish)
- TETRACAINE (MART.)
- TETRACAINE [MART.]
- TETRACAINE (USP-RS)
- TETRACAINE [USP-RS]
- Tetracaine (USP:INN:BAN)
- TETRACAINE (EP MONOGRAPH)
- TETRACAINE [EP MONOGRAPH]
- TETRACAINE (USP MONOGRAPH)
- TETRACAINE [USP MONOGRAPH]
- 100311-22-6
- Amethocaine (TN)
- Tetracaine (USP/INN)
- NCGC00016049-02
- CAS-136-47-0
- Landocaine
- Tetracain
- Tetracaine cream
- 2-Dimethylaminoethylester kyseliny p-butylaminobenzoove [Czech]
- FranklyNumb 3
- Diaethylaminoaethanol ester der p-butylaminobenzoesaeure [German]
- TE4
- Burn Solution Cream
- Spectrum_001032
- Pantocaine (Salt/Mix)
- Numfast Tetracaine Green
- TETRACAINE [INN]
- Prestwick0_000571
- Prestwick1_000571
- Prestwick2_000571
- Prestwick3_000571
- Spectrum2_001328
- Spectrum3_000562
- Spectrum4_000351
- Spectrum5_001072
- Lopac-T-7508
- TETRACAINE [VANDF]
- Epitope ID:174843
- BLT 3
- TETRACAINE [WHO-DD]
- Lopac0_001211
- SCHEMBL34714
- BSPBio_000382
- BSPBio_001944
- KBioGR_000781
- KBioSS_001512
- DivK1c_000607
- SPBio_001455
- SPBio_002601
- BPBio1_000422
- TETRACAINE [GREEN BOOK]
- Tetracaine, >=98% (TLC)
- DTXCID9023883
- TETRACAINE [ORANGE BOOK]
- KBio1_000607
- KBio2_001512
- KBio2_004080
- KBio2_006648
- KBio3_001444
- C05AD02
- D04AB06
- N01BA03
- S01HA03
- NINDS_000607
- BCPP000048
- ALBB-025902
- BCP04777
- HY-A0079
- BDBM50017659
- STL483844
- TETRACAINE COMPONENT OF SYNERA
- AKOS015889234
- AC-3480
- CCG-205285
- DB09085
- SDCCGSBI-0051178.P005
- IDI1_000607
- NCGC00016049-01
- NCGC00016049-03
- NCGC00016049-04
- NCGC00016049-14
- NCGC00162367-01
- AS-81743
- DA-78348
- SY066710
- BCP0726000001
- SBI-0051178.P004
- AB00053549
- NS00008930
- T2789
- Tetracaine, meets USP testing specifications
- .beta.-Dimethylaminoethyl p-butylaminobenzoate
- C07526
- D00551
- 2-(Dimethylamino)ethyl 4-(butylamino)benzoate #
- AB00053549-11
- AB00053549_12
- AB00053549_13
- EN300-6748839
- Q419608
- aniline, N-butyl-4-(2-dimethylaminoethoxycarbonyl)-
- Diathylaminoathanol ester der p-butylaminobenzosaure
- Q-201805
- BRD-K45071273-003-05-8
- BRD-K45071273-003-15-7
- BRD-K45071273-003-24-9
- BRD-K45071273-003-25-6
- Tetracaine, United States Pharmacopeia (USP) Reference Standard
- benzoic acid, 4-(butylamino)-, 2-(dimethylamino)ethyl ester ,hydrochloride
- BENZOIC ACID,4-BUTYLAMINO,2-DIMETHYLAMINOETHYL ESTER PANTOCAIN BASE
- InChI=1/C15H24N2O2/c1-4-5-10-16-14-8-6-13(7-9-14)15(18)19-12-11-17(2)3/h6-9,16H,4-5,10-12H2,1-3H
158.41 Ų [M+H-H2O]+ [CCS Type: TW; Method: calibrated with polyalanine and drug standards]
164.78 Ų [M+K]+ [CCS Type: TW; Method: calibrated with polyalanine and drug standards]
170.85 Ų [M+H]+ [CCS Type: TW; Method: calibrated with polyalanine and drug standards]
176.106 100
120.0436 25.89
177.1091 10.40
118.0643 6.15
148.1108 3.39
176.1062 100
177.1092 12.11
120.0437 8.78
148.1109 2.16
220.1321 1.90
265.1914 999
176.1064 952
266.1941 183
177.1094 118
220.1327 54
176.107 999
220.1327 155
221.1356 21
178.1118 11
265.1903 9
Tetracaine Hydrochloride (has salt form)
- Lidocaine; Tetracaine (component of)
- Benzocaine; Lidocaine; Tetracaine (component of)
- Lidocaine; tetracaine; witch hazel (component of)
N - Nervous system
N01 - Anesthetics
N01B - Anesthetics, local
N01BA - Esters of aminobenzoic acid
N01BA03 - Tetracaine
S - Sensory organs
S01 - Ophthalmologicals
S01H - Local anesthetics
S01HA - Local anesthetics
S01HA03 - Tetracaine
Use (kg; approx.) in Germany (2009): >50
Consumption (g per capita; approx.) in Germany (2009): 0.000611
Calculated removal (%): 44.3



H301 (97.6%): Toxic if swallowed [Danger Acute toxicity, oral]
H317 (92.9%): May cause an allergic skin reaction [Warning Sensitization, Skin]
H351 (92.9%): Suspected of causing cancer [Warning Carcinogenicity]
P203, P261, P264, P270, P272, P280, P301+P316, P302+P352, P318, P321, P330, P333+P317, P362+P364, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 42 reports by companies from 4 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Acute Tox. 3 (97.6%)
Skin Sens. 1 (92.9%)
Carc. 2 (92.9%)
IMAP assessments - Benzoic acid, 4-(butylamino)-, 2-(dimethylamino)ethyl ester: Environment tier I assessment
IMAP assessments - Benzoic acid, 4-(butylamino)-, 2-(dimethylamino)ethyl ester: Human health tier I assessment
◉ Summary of Use during Lactation
No information is available on the use of tetracaine during breastfeeding. Based on the low excretion of other local anesthetics into breastmilk, a single dose of injected tetracaine during breastfeeding, such as for a dental procedure, is unlikely to adversely affect the breastfed infant. However, an alternate drug may be preferred, especially while nursing a newborn or preterm infant. Topical application of tetracaine to the mother is unlikely to affect her breastfed infant if it is applied away from the breast. Only water-miscible cream or gel products should be applied to the breast because ointments may expose the infant to high levels of mineral paraffins via licking.
◉ Effects in Breastfed Infants
Relevant published information was not found as of the revision date.
◉ Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=GKCBAIGFKIBETG-UHFFFAOYSA-N
- Australian Industrial Chemicals Introduction Scheme (AICIS)Benzoic acid, 4-(butylamino)-, 2-(dimethylamino)ethyl esterhttps://services.industrialchemicals.gov.au/search-assessments/
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusTetracaine [USP:INN:BAN]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000094246ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_useTetracainehttps://www.drugbank.ca/drugs/DB09085
- EPA DSSToxCompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeTetracaine (EC: 202-316-6)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/15296
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- CCSbaseCCSbase Classificationhttps://ccsbase.net/
- ChEBI
- FDA Pharm ClassesLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingFDA Pharmacological Classificationhttps://www.fda.gov/ForIndustry/DataStandards/StructuredProductLabeling/ucm162549.htm
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licence
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- IUPAC Digitized pKa Datasetaniline, N-butyl-4-(2-dimethylaminoethoxycarbonyl)-https://github.com/IUPAC/Dissociation-Constants
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jsp
- Drug Gene Interaction database (DGIdb)LICENSEThe data used in DGIdb is all open access and where possible made available as raw data dumps in the downloads section.http://www.dgidb.org/downloadsTETRACAINEhttps://www.dgidb.org/drugs/rxcui:10391
- Therapeutic Target Database (TTD)
- Haz-Map, Information on Hazardous Chemicals and Occupational DiseasesLICENSECopyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://haz-map.com/AboutTetracainehttps://haz-map.com/Agents/1261
- DailyMed
- Drugs and Lactation Database (LactMed)
- Drugs@FDALICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/TETRACAINENORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- WHO Model Lists of Essential MedicinesLICENSEPermission from WHO is not required for the use of WHO materials issued under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Intergovernmental Organization (CC BY-NC-SA 3.0 IGO) license.https://www.who.int/about/policies/publishing/copyright
- EU Clinical Trials Register
- FDA Orange BookLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- National Drug Code (NDC) DirectoryLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingHMDB0258868_msms_2236109https://hmdb.ca/metabolites/HMDB0258868#spectra
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawTetracainehttp://www.nist.gov/srd/nist1a.cfm
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlAnatomical Therapeutic Chemical (ATC) classificationhttp://www.genome.jp/kegg-bin/get_htext?br08303.kegTarget-based classification of drugshttp://www.genome.jp/kegg-bin/get_htext?br08310.keg
- MassBank Europe
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- Metabolomics Workbench
- NIPH Clinical Trials Search of Japan
- SpectraBase4-Butylamino-benzoic acid, 2-dimethylamino-ethyl esterhttps://spectrabase.com/spectrum/8ChMOwW8iPZTETRACAINE HYDROCHLORIDE U.S.P.https://spectrabase.com/spectrum/32CN4mbDBpO
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.html
- NMRShiftDB
- WHO Anatomical Therapeutic Chemical (ATC) ClassificationLICENSEUse of all or parts of the material requires reference to the WHO Collaborating Centre for Drug Statistics Methodology. Copying and distribution for commercial purposes is not allowed. Changing or manipulating the material is not allowed.https://www.whocc.no/copyright_disclaimer/
- PharmGKBLICENSEPharmGKB data are subject to the Creative Commons Attribution-ShareALike 4.0 license (https://creativecommons.org/licenses/by-sa/4.0/).https://www.pharmgkb.org/page/policies
- PharosLICENSEData accessed from Pharos and TCRD is publicly available from the primary sources listed above. Please respect their individual licenses regarding proper use and redistribution.https://pharos.nih.gov/about
- Protein Data Bank in Europe (PDBe)
- RCSB Protein Data Bank (RCSB PDB)LICENSEData files contained in the PDB archive (ftp://ftp.wwpdb.org) are free of all copyright restrictions and made fully and freely available for both non-commercial and commercial use. Users of the data should attribute the original authors of that structural data.https://www.rcsb.org/pages/policies
- Springer Nature
- SpringerMaterialsBenzoic acid, 4-(butylamino)-, 2-(dimethylamino)ethyl esterhttps://materials.springer.com/substanceprofile/docs/smsid_ikqgjwlotdzebqxe
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidatatetracainehttps://www.wikidata.org/wiki/Q419608
- WikipediaOxybuprocainehttps://en.wikipedia.org/wiki/OxybuprocaineTetracainehttps://en.wikipedia.org/wiki/Tetracaine
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlAnesthetics, Localhttps://www.ncbi.nlm.nih.gov/mesh/68000779
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403383647https://pubchem.ncbi.nlm.nih.gov/substance/403383647
- NCBI