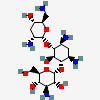
Tobramycin
- tobramycin
- 32986-56-4
- Nebramycin VI
- Nebramycin 6
- Nebramycin factor 6
- Create:2005-03-27
- Modify:2025-01-18
 Tobramycin Sulfate (has salt form); Dexamethasone; tobramycin (component of); Loteprednol etabonate; tobramycin (component of) ... View More ...
Tobramycin Sulfate (has salt form); Dexamethasone; tobramycin (component of); Loteprednol etabonate; tobramycin (component of) ... View More ...
- Brulamycin
- Nebcin
- Nebicin
- Nebramycin Factor 6
- Obracin
- Sulfate, Tobramycin
- Tobracin
- Tobramycin
- Tobramycin Sulfate
- tobramycin
- 32986-56-4
- Nebramycin VI
- Nebramycin 6
- Nebramycin factor 6
- Tobi
- Tobramicin
- 3'-Deoxykanamycin B
- Aktob
- Tobrex
- Tobi Podhaler
- Nebcin
- Deoxykanamycin B
- vantobra
- Tobramicina
- Tobramycine
- Tobralex
- Tobramycin Base
- 1-Epitobramycin
- Tobramycetin
- Tobramycinum
- Gotabiotic
- Tobradistin
- Tobrased
- Bethkis
- Tobacin
- Kitabis Pak
- Tobramitsetin
- Tenebrimycin
- Tenemycin
- Tobramaxin
- TOBRAMYCIN SULFATE
- NEBRAMYCIN
- Distobram
- Nebramycin factir 6
- Kitabis
- NSC 180514
- HSDB 3259
- UNII-VZ8RRZ51VK
- VZ8RRZ51VK
- Tobramycine [INN-French]
- Tobramycinum [INN-Latin]
- Tobramicina [INN-Spanish]
- EINECS 251-322-5
- Lilly 47663
- Tobracin (TN)
- Tobrex (TN)
- BRN 1357507
- Tobramycin Deuterated
- DTXSID8023680
- CHEBI:28864
- (2S,3R,4S,5S,6R)-4-amino-2-[(1S,2S,3R,4S,6R)-4,6-diamino-3-[(2R,3R,5S,6R)-3-amino-6-(aminomethyl)-5-hydroxyoxan-2-yl]oxy-2-hydroxycyclohexyl]oxy-6-(hydroxymethyl)oxane-3,5-diol
- NSC-180514
- DTXCID903680
- Obramycin
- ZYLET COMPONENT TOBRAMYCIN
- TOBRADEX COMPONENT TOBRAMYCIN
- TOBRASONE COMPONENT TOBRAMYCIN
- TOBRADEX ST COMPONENT TOBRAMYCIN
- Tobramycin [USAN:USP:INN:BAN:JAN]
- Nebicin
- Tobramycine (INN-French)
- Tobramycinum (INN-Latin)
- Tobramicina (INN-Spanish)
- TOBRAMYCIN (MART.)
- TOBRAMYCIN [MART.]
- TOBRAMYCIN (USP-RS)
- TOBRAMYCIN [USP-RS]
- (2S,3R,4S,5S,6R)-4-amino-2-(((1S,2S,3R,4S,6R)-4,6-diamino-3-(((2R,3R,5S,6R)-3-amino-6-(aminomethyl)-5-hydroxytetrahydro-2H-pyran-2-yl)oxy)-2-hydroxycyclohexyl)oxy)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,5-diol
- O-3-Amino-3-deoxy-alpha-D-glucopyranosyl-(1-4)-O-(2,6-diamino-2,3,6-trideoxy-alpha-D-ribo-hexopyranosyl-(1-6))-2-deoxy-L-streptamine
- O-3-Amino-3-deoxy-alpha-D-glucopyranosyl-(1-6)-O-(2,6-diamino-2,3,6-trideoxy-alpha-D-ribohexopyranosyl-(1-4))-2-deoxy-D-streptamine
- C18H37N5O9
- TOBRAMYCIN (EP MONOGRAPH)
- TOBRAMYCIN [EP MONOGRAPH]
- TOBRAMYCIN (USP MONOGRAPH)
- TOBRAMYCIN [USP MONOGRAPH]
- Tobramycin (USAN:USP:INN:BAN:JAN)
- (1S,2S,3R,4S,6R)-4,6-diamino-3-[(2,6-diamino-2,3,6-trideoxy-alpha-D-ribo-hexopyranosyl)oxy]-2-hydroxycyclohexyl 3-amino-3-deoxy-alpha-D-glucopyranoside
- (2S,3R,4S,5S,6R)-4-amino-2-{[(1S,2S,3R,4S,6R)-4,6-diamino-3-{[(2R,3R,5S,6R)-3-amino-6-(aminomethyl)-5-hydroxyoxan-2-yl]oxy}-2-hydroxycyclohexyl]oxy}-6-(hydroxymethyl)oxane-3,5-diol
- O-3-Amino-3-deoxy-alpha-D-glucopyranosyl-(1-4)-O-(2,6-diamino-2,3,6-trideoxy-alpha-D-ribohexopyranosyl-(1-4))-2-deoxy-D-streptamine
- TOY
- SR-05000001726
- MFCD00077885
- SPRC-AB01
- torbamycin
- Nebramycin Factor 6;Deoxykanamycin B
- Tobramycin,(S)
- Nebcin (Sulfate)
- NCGC00016814-01
- (2S,3R,4S,5S,6R)-4-amino-2-((1S,2S,3R,4S,6R)-4,6-diamino-3-((2R,3R,5S,6R)-3-amino-6-(aminomethyl)-5-hydroxytetrahydro-2H-pyran-2-yloxy)-2-hydroxycyclohexyloxy)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,5-diol
- D-STREPTAMINE, O-3-AMINO-3-DEOXY-.ALPHA.-D-GLUCOPYRANOSYL-(1->6)-O-(2,6-DIAMINO-2,3,6-TRIDEOXY-.ALPHA.-D-RIBO-HEXOPYRANOSYL-(1->4))-2-DEOXY-
- D-Streptamine, O-3-amino-3-deoxy-.alpha.-D-glucopyranosyl-(1->6)-O-[2,6-diamino-2,3,6-trideoxy-.alpha.-D-ribo-hexopyranosyl-(1->4)]-2-deoxy-
- O-3-Amino-3-deoxy-alpha-D-glucopyranosyl-(1-6)-O-[2,6-diamino-2,3,6-trideoxy-alpha-D-ribohexopyranosyl-(1-4)]-2-deoxy-D-streptamine
- Bethkis (TN)
- CAS-32986-56-4
- Tobramycin Inhalation
- Tobramycinum (Latin)
- AT02 - Tobramycin
- D-Streptamine, O-3-amino-3-deoxy-alpha-D-glucopyranosyl-(1-6)-O-(2,6-diamino-2,3,6-trideoxy-alpha-D-ribo-hexopyranosyl-(1-4))-2-deoxy-
- Tobi (TN)
- Spectrum_001072
- TOBRAMYCIN [MI]
- TOBRAMYCIN [INN]
- TOBRAMYCIN [JAN]
- Prestwick3_000544
- Spectrum2_000078
- Spectrum3_000588
- Spectrum4_000752
- Spectrum5_001038
- TOBRAMYCIN [HSDB]
- TOBRAMYCIN [USAN]
- TOBRAMYCIN [VANDF]
- Tobramycin (JP18/USP)
- SCHEMBL2838
- CHEMBL1747
- TOBRAMYCIN [WHO-DD]
- BSPBio_000587
- BSPBio_002036
- KBioGR_001104
- KBioSS_001552
- 4-(2,6-Diamino-2,3,6-trideoxy-alpha-D-glycopyranosyl)-6-(3-amino-3-deoxy-alpha-D-glycopyranosyl)-2-deoxystreptamine
- MLS000069544
- Streptamine, O-3-amino-3-deoxy-alpha-D-glucopyranosyl-(1-4)-O-(2,6-diamino-2,3,6-tyrideoxy-alpha-D-ribohexopyranosyl-(1-6))-2-deoxy-, D-
- BIDD:GT0503
- SPECTRUM1500579
- Tobramycin Inhalation Solution
- Tobramycin Ophthalmic Solution
- SPBio_000295
- BPBio1_000647
- TOBRAMYCIN [ORANGE BOOK]
- GTPL10930
- KBio2_001552
- KBio2_004120
- KBio2_006688
- KBio3_001536
- TobramycinInhalation Solution Pak
- J01GB01
- S01AA12
- HMS2090B16
- HMS2092M17
- HMS2096N09
- HMS3713N09
- Pharmakon1600-01500579
- HY-B0441
- TOBRAMYCIN COMPONENT OF ZYLET
- Tox21_110626
- BDBM50366778
- CCG-39936
- HB4564
- NSC757352
- s2514
- Tobradex (tobramycin + dexamethasone)
- AKOS016339662
- DB00684
- KS-1405
- NSC-757352
- TOBRAMYCIN COMPONENT OF TOBRADEX
- TOBRAMYCIN COMPONENT OF TOBRASONE
- NCGC00178852-01
- NCGC00178852-02
- (2S,3R,4S,5S,6R)-4-amino-2-[(1S,2S,3R,4S,6R)-4,6-diamino-3-[(2R,3R,5S,6R)-3-amino-6-(aminomethyl)-5-hydroxy-tetrahydropyran-2-yl]oxy-2-hydroxy-cyclohexoxy]-6-(hydroxymethyl)tetrahydropyran-3,5-diol
- SMR000058793
- Streptamine, O-3-amino-3-deoxy-alpha-D-glucopyranosyl-(1-4)-O-(2,6-diamino-2,3,6-trideoxy-alpha-D-ribohexopyranosyl-(1-6))-2-deoxy-, D-
- SBI-0051915.P003
- TOBRAMYCIN COMPONENT OF TOBRADEX ST
- AB00513858
- NS00003131
- T2503
- C00397
- D00063
- AB00052438-12
- AB00052438_13
- AB00052438_14
- EN300-7480857
- SR-01000721898
- Tobramycin, Antibiotic for Culture Media Use Only
- Q-201837
- Q1758380
- SR-01000721898-2
- SR-05000001726-1
- SR-05000001726-2
- BRD-K05619559-001-10-1
- BRD-K05619559-001-12-7
- (1S,2S,3R,4S,6R)-4,6-diamino-3-(2,6-diamino-2,3,6-trideoxy-alpha-D-ribo-hexopyranosyloxy)-2-hydroxycyclohexyl 3-amino-3-deoxy-alpha-D-glucopyranoside
- D-Streptamine, O-3-amino-3-deoxy-.alpha.-D-glucopyranosyl-(1->6)-O-[2,6-diamino-2,3,6-trideoxy-.alpha.-D-ribohexopyranosyl-(1->4)]-2-deoxy-
- D-Streptamine, O-3-amino-3-deoxy-.alpha.-D-glucopyranosyl-(1?6)-O-[2,6-diamino-2,3,6-trideoxy-.alpha.-D-ribo-hexopyranosyl-(1?4)]-2-deoxy-
- O-[3-Amino-3-deoxy-alpha-D-glucopyranosyl-(1-->6)]-O-[2,6-diamino-2,3,6-trideoxy-alpha-D-ribohexopyranosyl-(1-->4)]-2-deoxy-D-streptamine
- O-[3-Amino-3-deoxy-alpha-D-glucopyranosyl-(1->6)]-O-[2,6-diamino-2,3,6-trideoxy-alpha-D-ribo-hexopyranosyl-(1->4)]-2-deoxy-D-streptamine
- O-3-AMINO-3-DEOXY-.ALPHA.-D-GLUCOPYRANOSYL-(1->4)-O-(2,6-DIAMINO-2,3,6-TRIDEOXY-.ALPHA.-D-RIBO-HEXOPYRANOSYL-(1.ALPHA.6))-2-DEOXY-L-STREPTAMINE
- O-3-Amino-3-deoxy-a-D-glucopyranosyl-(1-6)-O-[2,6-diamino-2,3,6-trideoxy-a -D-ribo-hexopyranosyl-(1-4)]-2-deoxy-D-streptamine
- O-3-AMINO-3-DEOXY-alpha-D-GLUCOPYRANOSYL-(1->4)-O-(2,6-DIAMINO-2,3,6-TRIDEOXY-alpha-D-RIBO-HEXOPYRANOSYL-(1alpha6))-2-DEOXY-L-STREPTAMINE
57.070629 100
344.828424 99.76
312.838631 94.40
353.841149 64.44
67.054904 54.91
353.841111 100
336.838660 58.97
57.070613 51.36
394.844046 35.75
354.847208 22.01
152.06334433425897 0.04
207.03408633425897 0.02
128.06546433425896 0.02
404.28179967967037 0.02
158.09767333425896 0.02
Tobramycin Sulfate (has salt form)
- Dexamethasone; tobramycin (component of)
- Loteprednol etabonate; tobramycin (component of)
- Fluorometholone acetate; tobramycin (component of)
Use (kg; approx.) in Germany (2009): >100
Use (kg; exact) in Germany (2009): 218
Use (kg) in USA (2002): 394
Consumption (g per capita; approx.) in Germany (2009): 0.00122
Consumption (g per capita; exact) in Germany (2009): 0.00266
Consumption (g per capita) in the USA (2002): 0.0014
Calculated removal (%): 92.1



H315 (50%): Causes skin irritation [Warning Skin corrosion/irritation]
H317 (14.3%): May cause an allergic skin reaction [Warning Sensitization, Skin]
H319 (42.9%): Causes serious eye irritation [Warning Serious eye damage/eye irritation]
H335 (42.9%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation]
H360 (21.4%): May damage fertility or the unborn child [Danger Reproductive toxicity]
H361 (14.3%): Suspected of damaging fertility or the unborn child [Warning Reproductive toxicity]
H400 (14.3%): Very toxic to aquatic life [Warning Hazardous to the aquatic environment, acute hazard]
P203, P261, P264, P264+P265, P271, P272, P273, P280, P302+P352, P304+P340, P305+P351+P338, P318, P319, P321, P332+P317, P333+P317, P337+P317, P362+P364, P391, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 14 reports by companies from 12 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Reported as not meeting GHS hazard criteria per 1 of 14 reports by companies. For more detailed information, please visit ECHA C&L website.
There are 11 notifications provided by 13 of 14 reports by companies with hazard statement code(s).
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Skin Irrit. 2 (50%)
Skin Sens. 1 (14.3%)
Eye Irrit. 2 (42.9%)
STOT SE 3 (42.9%)
Repr. 1B (21.4%)
Repr. 2 (14.3%)
Aquatic Acute 1 (14.3%)
Acute Tox. 3 (16.7%)
Acute Tox. 4 (16.7%)
Acute Tox. 3 (16.7%)
Acute Tox. 4 (50%)
Acute Tox. 3 (16.7%)
Acute Tox. 4 (50%)
Repr. 1B (50%)
Repr. 2 (33.3%)
Lact. (33.3%)
STOT RE 2 (16.7%)
Hazard Traits - Developmental Toxicity
Authoritative List - Prop 65
Report - regardless of intended function of ingredient in the product
◉ Summary of Use during Lactation
Tobramycin is poorly excreted into breastmilk. Newborn infants apparently absorb small amounts of other aminoglycosides, but serum levels with three times daily dosages are far below those attained when treating newborn infections and systemic effects of tobramycin are unlikely. Older infants would be expected to absorb even less tobramycin. Because there is little variability in the milk tobramycin levels during multiple daily dose regimens, timing breastfeeding with respect to the dose is of little or no benefit in reducing infant exposure. Data are not available with single daily dose regimens. Monitor the infant for possible effects on the gastrointestinal flora, such as diarrhea, candidiasis (e.g., thrush, diaper rash) or rarely, blood in the stool indicating possible antibiotic-associated colitis.
Maternal use of an ear drop or eye drop that contains tobramycin presents little or no risk for the nursing infant. A task force respiratory experts from Europe, Australia and New Zealand found that inhaled tobramycin is compatible with breastfeeding.
◉ Effects in Breastfed Infants
An infant was breastfed (extent not stated) until the 4th month postpartum. At 2 months of age, his mother was given a 2-week course of tobramycin 150 mg three times daily plus meropenem for a cystic fibrosis exacerbation. infant displayed no change in stool pattern during the maternal treatment and had normal renal function at 6 months of age.
◉ Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=NLVFBUXFDBBNBW-PBSUHMDJSA-N
- California Office of Environmental Health Hazard Assessment (OEHHA)Tobramycin Sulfatehttps://oehha.ca.gov/proposition-65/chemicals/tobramycin-sulfate
- ChEBI
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_useTobramycinhttps://www.drugbank.ca/drugs/DB00684
- FDA Pharm ClassesLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingFDA Pharmacological Classificationhttps://www.fda.gov/ForIndustry/DataStandards/StructuredProductLabeling/ucm162549.htm
- LiverTox
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/Tobramycinhttps://www.wikidata.org/wiki/Q1758380LOTUS Treehttps://lotus.naturalproducts.net/
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licence
- California Safe Cosmetics Program (CSCP) Product Database
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusTobramycin [USAN:USP:INN:BAN:JAN]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0032986564ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DTP/NCILICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuse
- EPA DSSToxCompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-notice(2S,3R,4S,5S,6R)-4-amino-2-{[(1S,2S,3R,4S,6R)-4,6-diamino-3-{[(2R,3R,5S,6R)-3-amino-6-(aminomethyl)-5-hydroxyoxan-2-yl]oxy}-2-hydroxycyclohexyl]oxy}-6-(hydroxymethyl)oxane-3,5-diolhttps://echa.europa.eu/substance-information/-/substanceinfo/100.120.947Tobramycin (EC: 251-322-5)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/50835(2S,3R,4S,5S,6R)-4-amino-2-{[(1S,2S,3R,4S,6R)-4,6-diamino-3-{[(2R,3R,5S,6R)-3-amino-6-(aminomethyl)-5-hydroxyoxan-2-yl]oxy}-2-hydroxycyclohexyl]oxy}-6-(hydroxymethyl)oxane-3,5-diol (EC: 616-717-2)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/73223
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Hazardous Substances Data Bank (HSDB)
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citing
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jsp
- Drug Gene Interaction database (DGIdb)LICENSEThe data used in DGIdb is all open access and where possible made available as raw data dumps in the downloads section.http://www.dgidb.org/downloadsTOBRAMYCINhttps://www.dgidb.org/drugs/rxcui:10627
- IUPHAR/BPS Guide to PHARMACOLOGYLICENSEThe Guide to PHARMACOLOGY database is licensed under the Open Data Commons Open Database License (ODbL) https://opendatacommons.org/licenses/odbl/. Its contents are licensed under a Creative Commons Attribution-ShareAlike 4.0 International License (http://creativecommons.org/licenses/by-sa/4.0/)https://www.guidetopharmacology.org/about.jsp#licenseGuide to Pharmacology Target Classificationhttps://www.guidetopharmacology.org/targets.jsp
- Therapeutic Target Database (TTD)
- DailyMed
- European Medicines Agency (EMA)LICENSEInformation on the European Medicines Agency's (EMA) website is subject to a disclaimer and copyright and limited reproduction notices.https://www.ema.europa.eu/en/about-us/legal-noticeVantobra (previously Tobramycin PARI) (EMEA/H/C/005086)https://www.ema.europa.eu/en/medicines/human/EPAR/vantobra-previously-tobramycin-pariTobi Podhaler (EMEA/H/C/002155)https://www.ema.europa.eu/en/medicines/human/EPAR/tobi-podhalerVantobra (EMEA/H/C/002633)https://www.ema.europa.eu/en/medicines/human/EPAR/vantobraTobramycin (P/0114/2017)https://www.ema.europa.eu/en/medicines/human/paediatric-investigation-plans/emea-000184-pip03-16Tobramycin (P/0184/2014)https://www.ema.europa.eu/en/medicines/human/paediatric-investigation-plans/emea-000184-pip02-14
- Drugs and Lactation Database (LactMed)
- Drugs@FDALICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- WHO Model Lists of Essential MedicinesLICENSEPermission from WHO is not required for the use of WHO materials issued under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Intergovernmental Organization (CC BY-NC-SA 3.0 IGO) license.https://www.who.int/about/policies/publishing/copyright
- EU Clinical Trials Register
- FDA Orange BookLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/TobramycinNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- WHO Anatomical Therapeutic Chemical (ATC) ClassificationLICENSEUse of all or parts of the material requires reference to the WHO Collaborating Centre for Drug Statistics Methodology. Copying and distribution for commercial purposes is not allowed. Changing or manipulating the material is not allowed.https://www.whocc.no/copyright_disclaimer/
- National Drug Code (NDC) DirectoryLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlTherapeutic category of drugs in Japanhttp://www.genome.jp/kegg-bin/get_htext?br08301.kegUSP drug classificationhttp://www.genome.jp/kegg-bin/get_htext?br08302.kegAnatomical Therapeutic Chemical (ATC) classificationhttp://www.genome.jp/kegg-bin/get_htext?br08303.kegAntiinfectiveshttp://www.genome.jp/kegg-bin/get_htext?br08307.kegDrugs listed in the Japanese Pharmacopoeiahttp://www.genome.jp/kegg-bin/get_htext?br08311.keg
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- Metabolomics Workbench
- Nature Chemical Biology
- NIPH Clinical Trials Search of Japan
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawTobramycinhttp://www.nist.gov/srd/nist1a.cfm
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.htmltobramycin sulfatehttps://rxnav.nlm.nih.gov/id/rxnorm/7276
- PharmGKBLICENSEPharmGKB data are subject to the Creative Commons Attribution-ShareALike 4.0 license (https://creativecommons.org/licenses/by-sa/4.0/).https://www.pharmgkb.org/page/policies
- Protein Data Bank in Europe (PDBe)
- RCSB Protein Data Bank (RCSB PDB)LICENSEData files contained in the PDB archive (ftp://ftp.wwpdb.org) are free of all copyright restrictions and made fully and freely available for both non-commercial and commercial use. Users of the data should attribute the original authors of that structural data.https://www.rcsb.org/pages/policies
- SpectraBase4,6-Diamino-3-[(3-amino-3-deoxyhexopyranosyl)oxy]-2-hydroxycyclohexyl 2,6-diamino-2,3,6-trideoxyhexopyranosidehttps://spectrabase.com/spectrum/1Gi59aVj8QITobramycinehttps://spectrabase.com/spectrum/4PwyNYDVdw
- Springer Nature
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidatatobramycinhttps://www.wikidata.org/wiki/Q1758380
- WikipediaHydralazinehttps://en.wikipedia.org/wiki/HydralazineTobramycinhttps://en.wikipedia.org/wiki/Tobramycin
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlAnti-Bacterial Agentshttps://www.ncbi.nlm.nih.gov/mesh/68000900
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- The Natural Products AtlasLICENSEThe Natural Products Atlas is licensed under a Creative Commons Attribution 4.0 International License.https://www.npatlas.org/termsThe Natural Products Atlas Classificationhttps://www.npatlas.org/
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403383245https://pubchem.ncbi.nlm.nih.gov/substance/403383245
- NCBI