Cloral betaine
- Cloral betaine
- CHLORAL BETAINE
- 2218-68-0
- Cloral betaina
- Chloral hydrate betaine (1:1) compound
- Create:2005-08-08
- Modify:2025-01-18
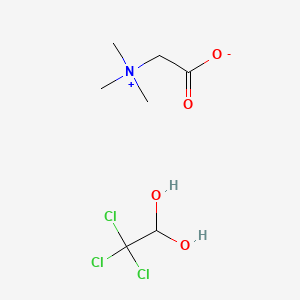
- Cloral betaine
- CHLORAL BETAINE
- 2218-68-0
- Cloral betaina
- Chloral hydrate betaine (1:1) compound
- Chloral betaine [USAN:NF]
- Cloral betaine [INN]
- 8680278NRH
- Betainchloralum
- Somnalchlor
- Somilan
- beta-Chlor
- Cloral betaina [INN-Spanish]
- 2,2,2-trichloroethane-1,1-diol;2-(trimethylazaniumyl)acetate
- Cloralum betainum
- Cloralum betainum [INN-Latin]
- EINECS 218-722-1
- DEA No. 2460
- UNII-8680278NRH
- MJ 5107
- CHLORAL BETAINE [MI]
- Chloral betaine (USAN/INN)
- SCHEMBL124513
- CHLORAL BETAINE [USAN]
- CLORAL BETAINE [MART.]
- CHEMBL3833335
- CLORAL BETAINE [WHO-DD]
- DTXSID20176716
- ONAOIDNSINNZOA-UHFFFAOYSA-N
- DB01494
- NS00067965
- D03459
- Q5102907

H302 (100%): Harmful if swallowed [Warning Acute toxicity, oral]
H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation]
H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation]
P264, P264+P265, P270, P280, P301+P317, P302+P352, P305+P351+P338, P321, P330, P332+P317, P337+P317, P362+P364, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=ONAOIDNSINNZOA-UHFFFAOYSA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/Chloral Betainehttps://commonchemistry.cas.org/detail?cas_rn=2218-68-0
- ChemIDplusChloral betaine [USAN:NF]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0002218680ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_useChloral betainehttps://www.drugbank.ca/drugs/DB01494
- EPA DSSToxChloral betaine [USAN:NF]https://comptox.epa.gov/dashboard/DTXSID20176716CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeCloral betaine (EC: 218-722-1)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/122204
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.html
- Drug Enforcement Administration (DEA)LICENSEUnless otherwise indicated, information on Department of Justice websites is in the public domain and may be copied and distributed without permission. Citation of the Department of Justice as source of the information is appreciated, as appropriate.https://www.justice.gov/legalpoliciesChloral betainehttps://www.deadiversion.usdoj.gov/schedules/DEA drug and chemical classificationhttps://www.dea.gov/drug-information/drug-scheduling
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licenceCHLORAL BETAINEhttps://platform.opentargets.org/drug/CHEMBL3833335
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.html
- Metabolomics Workbench
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/Chloral betaine [USAN:NF]NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- SpectraBaseChloral betainehttps://spectrabase.com/spectrum/KZhSeSSXzW4Chloral .beta.inehttps://spectrabase.com/spectrum/BNlgtObovnVCHLORAL BETAINEhttps://spectrabase.com/spectrum/XTVVoRLf43
- Springer Nature
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidatachloral betainehttps://www.wikidata.org/wiki/Q5102907
- WikipediaChloral betainehttps://en.wikipedia.org/wiki/Chloral_betaine
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403747827https://pubchem.ncbi.nlm.nih.gov/substance/403747827
- NCBI
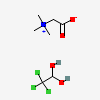
 CID 248 (N,N,N-trimethylglycinium)
CID 248 (N,N,N-trimethylglycinium) CID 2707 (Chloral Hydrate)
CID 2707 (Chloral Hydrate)