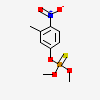
Fenitrothion
- FENITROTHION
- 122-14-5
- Phenitrothion
- Methylnitrophos
- Sumithion
- Create:2005-03-27
- Modify:2025-01-18

- Agria 1050
- Fenitrothion
- Folithion
- Metathion
- Methylnitrophos
- Nitrophos
- Sumithion
- Sumithione
- FENITROTHION
- 122-14-5
- Phenitrothion
- Methylnitrophos
- Sumithion
- Oleosumifene
- Folithion
- Metathion
- Nitrophos
- Ovadofos
- Owadofos
- Owadophos
- Sumifene
- Verthion
- Accothion
- Agrothion
- Cekutrothion
- Falithion
- Metathione
- Metathionine
- Metation
- Novathion
- Akotion
- Arbogal
- Fenitox
- Kotion
- Macbar
- Nuvanol
- Cyfen
- Cytel
- Mglawik F
- Folithion ec 50
- MEP (Pesticide)
- Super Sumithion
- Pennwalt C-4852
- Agriya 1050
- O,O-Dimethyl O-4-nitro-m-tolyl phosphorothioate
- Sumitomo S-1102A
- Fentrothione
- Oleometathion
- Fenition
- Sumigran
- Agria 1050
- Bayer S 5660
- Insectigas F
- Metathionine E50
- Metation E50
- Monsanto CP 47114
- Metathion E 50
- Bayer 41831
- Fenitrothion [BAN]
- Fenitrotion
- Aceothion
- American cyanamid CL-47,300
- Dicofen
- Sumithian
- Tionfos 50 LE
- Cyten
- Caswell No. 373
- Phosphorothioic acid, O,O-dimethyl O-(3-methyl-4-nitrophenyl) ester
- Fenitrotion (Hungarian)
- S 112A
- OMS 43
- O,O-Dimethyl O-(3-methyl-4-nitrophenyl) phosphorothioate
- Metathion E-50
- Metathio E-50
- Metation E-50
- O,O-Dimethyl O-(3-methyl-4-nitrophenyl) thiophosphate
- Metathionine E-50
- Dimethyl 4-nitro-m-tolyl phosphorothionate
- BAY 41831
- CCRIS 1559
- ENT 25,715
- S-1102A
- HSDB 1590
- AC-47300
- CL 47300
- CP 47114
- Dimethyl 3-methyl-4-nitrophenyl phosphorothionate
- EI 47300
- Fenitrothion [BSI:ISO]
- UNII-W8M4X3Y7ZY
- O,O-Dimethyl O-(4-nitro-3-methylphenyl)thiophosphate
- EINECS 204-524-2
- W8M4X3Y7ZY
- 8057HC
- MEP (Phosphorus insecticide)
- EPA Pesticide Chemical Code 105901
- BRN 1887367
- DTXSID4032613
- CHEBI:34757
- AI3-25715
- O,O-Dimetil-O-(3-metil-4-nitro-fenil)-monotiofosfato
- O,O-Dimethyl-O-(3-methyl-4-nitrofenyl)-monothiofosfaat
- Dimethyl-(3-methyl-4-nitrophenyl)thiophosphate
- C-9146
- FENITROTHION [MI]
- Phosphorothioic acid, O,O-dimethyl O-(4-nitro-m-tolyl) ester
- 3-Methyl-4-nitrophenyl dimethyl phosphorothioate
- Dimethyl (3-methyl-4-nitrophenyl) thiophosphate
- FENITROTHION [ISO]
- m-Cresol, 4-nitro-, O-ester with O,O-dimethyl phosphorothioate
- FENITROTHION [HSDB]
- FENITROTHION [MART.]
- O,O-Dimethyl O-(4-nitro-3-methylphenyl) thiophosphate
- O,O-Dimethyl O-(3-methyl-4-nitrophenyl) phosphorothionate
- DTXCID2012613
- O,O-Dimethyl O-4-nitro-m-tolyl thiophosphate
- O,O-Dimethyl-O-(3-methyl-4-nitrofenyl)-monothiofosfaat [Dutch]
- O,O-Dimethyl-O-(4-nitro-5-methylphenyl)-thionophosphat [German]
- O,O-Dimetil-O-(3-metil-4-nitro-fenil)-monotiofosfato [Italian]
- O,O-Dimetil-O-(3-metil-4-nitrofenil) fosforotioato (Portugese)
- Thiophosphate de O,O-dimethyle et de O-(3-methyl-4-nitrophenyle)
- S 5660
- BAY S 5660
- O,O-Dimethyl-O-(3-methyl-4-nitro-phenyl)-monothiophosphat [German]
- Tionofosforan O,O-dwumetylo-O-(3-metylo)-4-nitrofenylowy [Polish]
- O,O-DiMe O-(3-methyl-4-nitrophenyl) thiophosphate
- Thiophosphate de O,O-dimethyle et de O-(3-methyl-4-nitrophenyle) [French]
- O,O-Dimetil-O-(3-metil-4-nitrofenil) fosforotioato
- O,O-dimethyl O-(3-methyl-4-nitrophenyl)phosphorothioate
- O,O-Dimethyl-O-(4-nitro-5-methylphenyl)-thionophosphat
- O,O-Dimethyl-O-(4-nitro-5-methylphenyl)-thionophosphate
- O,O-Dimethyl-O-(3-methyl-4-nitro-phenyl)-monothiophosphat
- Tionofosforan O,O-dwumetylo-O-(3-metylo)-4-nitrofenylowy
- Fenitrothion 1000 microg/mL in Toluene
- Fenitrothion 10 microg/mL in Cyclohexane
- O,O-Dimethyl-O-(3-methyl-4-nitro-phenyl)-monothiophosphate
- Fenitrothion 100 microg/mL in Cyclohexane
- Fenitrothion 100 microg/mL in Acetonitrile
- FENITROTHION (MART.)
- O,O-DIMETHYL O-3-METHYL-4-NITROPHENYL PHOSPHOROTHIOATE
- methyl 3-methyl-4-nitrophenyl methoxy(sulfanylidene)phosphonite
- O,O-Dimethyl-(3-methyl-4-nitrophenyl) ester of phosphorothioic acid
- Sumithion 20F
- Sumithion 20MC
- Sumithion 50EC
- methadion
- O,O-Dimethyl-O-(3-methyl-4-nitro-phenyl)-monothiophosphat (German)
- O,O-Dimethyl-O-(3-methyl-4-nitrofenyl)-monothiofosfaat (Dutch)
- O,O-Dimetil-O-(3-metil-4-nitro-fenil)-monotiofosfato (Italian)
- Thiophosphate de O,O-dimethyle et de O-(3-methyl-4-nitrophenyle) (French)
- Fenitrothion (MEP)
- MFCD00055407
- METHATHION
- Pennwalt C4852
- Sumitomo S1102A
- MEP(PESTICIDE)
- Fenitrothion (Standard)
- METATION E 50
- METATHIONINE E 50
- SCHEMBL26916
- BIDD:ER0336
- American Cyanamid CL47,300
- CHEMBL347698
- HY-B1885R
- HY-B1885
- Tox21_300906
- S1102A
- AKOS015907800
- AKOS040744449
- Dimethyl 4nitromtolyl phosphorothionate
- 80557HC
- AC47300
- KS-5043
- NCGC00091902-04
- NCGC00166191-01
- NCGC00166191-02
- NCGC00166191-03
- NCGC00254810-01
- CAS-122-14-5
- Dimethyl(3methyl4nitrophenyl)thiophosphate
- O,ODimethyl O4nitromtolyl phosphorothioate
- AC 47,300
- DB-041652
- 3Methyl4nitrophenyl dimethyl phosphorothioate
- C9146
- CS-0013949
- Dimethyl (3methyl4nitrophenyl) thiophosphate
- NS00003255
- O,O-Dimethyl O-(3-methyl) phosphorothioate
- O,ODimetilO(3metil4nitrofenil)monotiofosfato
- Dimethyl 3methyl4nitrophenyl phosphorothionate
- H10437
- O,ODimethyl O(4nitro3methylphenyl)thiophosphate
- O,ODimethylO(3methyl4nitrofenyl)monothiofosfaat
- O,ODimethylO(4nitro5methylphenyl)thionophosphat
- Fenitrothion, PESTANAL(R), analytical standard
- O,ODimethyl O(3methyl4nitrophenyl) thiophosphate
- O,ODimethyl O(4nitro3methylphenyl) thiophosphate
- O,ODimethylO(3methyl4nitrophenyl)monothiophosphat
- A804852
- Q417614
- Tionofosforan O,OdwumetyloO(3metylo)4nitrofenylowy
- J-004767
- O,ODimethyl O(3methyl4nitrophenyl) phosphorothioate
- O,ODimethyl O(3methyl4nitrophenyl) phosphorothionate
- O,ODimetilO(3metil4nitrofenil) fosforotioato (Portugese)
- Phosphorothioic acid, O,Odimethyl O(4nitromtolyl) ester
- dimethoxy-(3-methyl-4-nitrophenoxy)-sulfanylidenephosphorane
- mCresol, 4nitro, Oester with O,Odimethyl phosphorothioate
- O,O-Dimethyl-O-(3-methyl-4-nitrophenyl)phosphorothionate
- Thiophosphate de O,Odimethyle et de O(3methyl4nitrophenyle)
- Phosphorothioic acid, O,Odimethyl O(3methyl4nitrophenyl) ester
- dimethoxy-(3-methyl-4-nitro-phenoxy)-sulfanylidene-$l^{5}-phosphane
- dimethoxy-(3-methyl-4-nitrophenoxy)-sulfanylidene-|E5-phosphane
- dimethoxy-(3-methyl-4-nitrophenoxy)-sulfanylidene-lambda5-phosphane
260.0 99.99
79.0 88.10
93.0 59.70
47.0 47.90
125.0 22.60
277.0 99.99
109.0 75.79
125.0 71.75
260.0 34.62
278.0 11.20
260 999
79 881
93 597
47 479
125 226
277 999
109 758
125 718
260 346
278 112


H302 (50.4%): Harmful if swallowed [Warning Acute toxicity, oral]
H312 (49.6%): Harmful in contact with skin [Warning Acute toxicity, dermal]
H400 (100%): Very toxic to aquatic life [Warning Hazardous to the aquatic environment, acute hazard]
H410 (55.6%): Very toxic to aquatic life with long lasting effects [Warning Hazardous to the aquatic environment, long-term hazard]
P264, P270, P273, P280, P301+P317, P302+P352, P317, P321, P330, P362+P364, P391, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 117 reports by companies from 5 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Acute Tox. 4 (50.4%)
Acute Tox. 4 (49.6%)
Aquatic Acute 1 (100%)
Aquatic Chronic 1 (55.6%)
Acute toxicity - category 4
Hazardous to the aquatic environment (acute) - category 1
Hazardous to the aquatic environment (chronic) - category 1
Warning: Effects may be delayed up to 12 hours. Caution is advised.
Note: Fenitrothion is a cholinesterase inhibitor.
Signs and Symptoms of Fenitrothion Exposure: Acute exposure to fenitrothion may produce the following signs and symptoms: sweating, pinpoint pupils, blurred vision, headache, dizziness, profound weakness, muscle spasms, seizures, and coma. Mental confusion and psychosis may occur. Excessive salivation, nausea, vomiting, anorexia, diarrhea, and abdominal pain may also occur. The heart rate may decrease following oral exposure or increase following dermal exposure. Chest pain may be noted. Hypotension (low blood pressure) may be observed, although hypertension (high blood pressure) is not uncommon. Respiratory symptoms include dyspnea (shortness of breath), pulmonary edema, respiratory depression, and respiratory paralysis.
Emergency Life-Support Procedures: Acute exposure to fenitrothion exposure may require decontamination and life support for the victims. Emergency personnel should wear protective clothing appropriate to the type and degree of contamination. Air-purifying or supplied-air respiratory equipment should also be worn, as necessary. Rescue vehicles should carry supplies such as plastic sheeting and disposable plastic bags to assist in preventing spread of contamination.
Inhalation Exposure:
1. Move victims to fresh air. Emergency personnel should avoid self-exposure to fenitrothion.
2. Evaluate vital signs including pulse and respiratory rate, and note any trauma. If no pulse is detected, provide CPR. If not breathing, provide artificial respiration. If breathing is labored, administer oxygen or other respiratory support.
3. Obtain authorization and/or further instructions from the local hospital for administration of an antidote or performance of other invasive procedures.
4. Transport to a health care facility.
Dermal/Eye Exposure:
1. Remove victims from exposure. Emergency personnel should avoid self-exposure to fenitrothion.
2. Evaluate vital signs including pulse and respiratory rate, and note any trauma. If no pulse is detected, provide CPR. If not breathing, provide artificial respiration. If breathing is labored, administer oxygen or other respiratory support.
3. Remove contaminated clothing as soon as possible.
4. If eye exposure has occurred, eyes must be flushed with lukewarm water for at least 15 minutes.
5. Wash exposed skin areas three times with soap and water.
6. Obtain authorization and/or further instructions from the local hospital for administration of an antidote or performance of other invasive procedures.
7. Transport to a health care facility.
Ingestion Exposure:
1. Evaluate vital signs including pulse and respiratory rate, and note any trauma. If no pulse is detected, provide CPR. If not breathing, provide artificial respiration. If breathing is labored, administer oxygen or other respiratory support.
2. Obtain authorization and/or further instructions from the local hospital for administration of an antidote or performance of other invasive procedures.
3. Vomiting may be induced with syrup of Ipecac. If elapsed time since ingestion of fenitrothion is unknown or suspected to be greater than 30 minutes, do not induce vomiting and proceed to Step
4. Ipecac should not be administered to children under 6 months of age.Warning: Ingestion of fenitrothion may result in sudden onset of seizures or loss of consciousness. Syrup of Ipecac should be administered only if victims are alert, have an active gag-reflex, and show no signs of impending seizure or coma. If ANY uncertainty exists, proceed to Step
4.The following dosages of Ipecac are recommended: children up to 1 year old, 10 mL (1/3 oz); children 1 to 12 years old, 15 mL (1/2 oz); adults, 30 mL (1 oz). Ambulate (walk) the victims and give large quantities of water. If vomiting has not occurred after 15 minutes, Ipecac may be readministered. Continue to ambulate and give water to the victims. If vomiting has not occurred within 15 minutes after second administration of Ipecac, administer activated charcoal.
4. Activated charcoal may be administered if victims are conscious and alert. Use 15 to 30 g (1/2 to 1 oz) for children, 50 to 100 g (1-3/4 to 3-1/2 oz) for adults, with 125 to 250 mL (1/2 to 1 cup) of water.
5. Promote excretion by administering a saline cathartic or sorbitol to conscious and alert victims. Children require 15 to 30 g (1/2 to 1 oz) of cathartic; 50 to 100 g (1-3/4 to 3- 1/2 oz) is recommended for adults.
6. Transport to a health care facility. (EPA, 1998)
(Non-Specific -- Organophosphate Pesticide n.o.s.) Move containers from fire area if you can do so without risk. Fight fire from maximum distance. Dike fire control water for later disposal; do not scatter the material. Wear positive pressure breathing apparatus and special protective clothing.
This compound is an organophosphate insecticide.
Small fires: dry chemical, carbon dioxide, water spray, or foam. Large fires: water spray, fog or foam. (EPA, 1998)
Excerpt from ERG Guide 131 [Flammable Liquids - Toxic]:
IMMEDIATE PRECAUTIONARY MEASURE: Isolate spill or leak area for at least 50 meters (150 feet) in all directions.
SPILL: Increase the immediate precautionary measure distance, in the downwind direction, as necessary.
FIRE: If tank, rail tank car or highway tank is involved in a fire, ISOLATE for 800 meters (1/2 mile) in all directions; also, consider initial evacuation for 800 meters (1/2 mile) in all directions. (ERG, 2024)
(Non-Specific -- Organophosphate Pesticide n.o.s.) Keep unnecessary people away; isolate hazard area and deny entry. Stay upwind; keep out of low areas. Ventilate closed spaces before entering them. Remove and isolate contaminated clothing at the site. Do not touch spilled material; stop leak if you can do so without risk. Use water spray to reduce vapors.
Small spills: absorb with sand or other noncombustible absorbent material and place into containers for later disposal.
Large spills: dike far ahead of spill for later disposal. (EPA, 1998)
Hazard Traits - Developmental Toxicity; Neurotoxicity
Authoritative List - CECBP - Priority Chemicals
Report - if used as a fragrance or flavor ingredient
IMAP assessments - Phosphorothioic acid, O,O-dimethyl O-(3-methyl-4-nitrophenyl) ester: Environment tier I assessment
IMAP assessments - Phosphorothioic acid, O,O-dimethyl O-(3-methyl-4-nitrophenyl) ester: Human health tier I assessment
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=ZNOLGFHPUIJIMJ-UHFFFAOYSA-N
- Australian Industrial Chemicals Introduction Scheme (AICIS)Phosphorothioic acid, O,O-dimethyl O-(3-methyl-4-nitrophenyl) esterhttps://services.industrialchemicals.gov.au/search-assessments/
- CAMEO ChemicalsLICENSECAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data.https://cameochemicals.noaa.gov/help/reference/terms_and_conditions.htm?d_f=falseFENITROTHIONhttps://cameochemicals.noaa.gov/chemical/4998CAMEO Chemical Reactivity Classificationhttps://cameochemicals.noaa.gov/browse/react
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxFenitrothionhttps://comptox.epa.gov/dashboard/DTXSID4032613CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- EPA Safe Drinking Water Act (SDWA)
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeFenitrothionhttps://chem.echa.europa.eu/100.004.114Fenitrothion (EC: 204-524-2)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/109266
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Hazardous Substances Data Bank (HSDB)
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingFenitrothionhttp://www.hmdb.ca/metabolites/HMDB0041893HMDB0041893_cms_28792https://hmdb.ca/metabolites/HMDB0041893#spectra
- ILO-WHO International Chemical Safety Cards (ICSCs)
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/
- Risk Assessment Information System (RAIS)LICENSEThis work has been sponsored by the U.S. Department of Energy (DOE), Office of Environmental Management, Oak Ridge Operations (ORO) Office through a joint collaboration between United Cleanup Oak Ridge LLC (UCOR), Oak Ridge National Laboratory (ORNL), and The University of Tennessee, Ecology and Evolutionary Biology, The Institute for Environmental Modeling (TIEM). All rights reserved.https://rais.ornl.gov/Fenitrothionhttps://rais.ornl.gov/cgi-bin/tools/TOX_search
- California Safe Cosmetics Program (CSCP) Product Database
- EU Pesticides Database
- Haz-Map, Information on Hazardous Chemicals and Occupational DiseasesLICENSECopyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://haz-map.com/AboutFenitrothionhttps://haz-map.com/Agents/5279
- ChEBI
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- Toxin and Toxin Target Database (T3DB)LICENSET3DB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (T3DB) and the original publication.http://www.t3db.ca/downloadsFenitrothionhttp://www.t3db.ca/toxins/T3D3839
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jsp
- Drug Gene Interaction database (DGIdb)LICENSEThe data used in DGIdb is all open access and where possible made available as raw data dumps in the downloads section.http://www.dgidb.org/downloadsFENITROTHIONhttps://www.dgidb.org/drugs/ncit:C65655
- EPA Pesticide Ecotoxicity Database
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/fenitrothionNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- USDA Pesticide Data ProgramFenitrothionhttps://www.ams.usda.gov/datasets/pdp
- Hazardous Chemical Information System (HCIS), Safe Work Australia
- NITE-CMCFenitrothion - FY2006 (New/original classication)https://www.chem-info.nite.go.jp/chem/english/ghs/06-imcg-0151e.htmlO,O-Dimethyl-O-(3-methyl-4-nitrophenyl) thiophosphate [Fenitrothion] - FY2017 (Revised classification)https://www.chem-info.nite.go.jp/chem/english/ghs/17-mhlw-2076e.html
- Regulation (EC) No 1272/2008 of the European Parliament and of the CouncilLICENSEThe copyright for the editorial content of this source, the summaries of EU legislation and the consolidated texts, which is owned by the EU, is licensed under the Creative Commons Attribution 4.0 International licence.https://eur-lex.europa.eu/content/legal-notice/legal-notice.htmlfenitrothion (ISO); O,O-dimethyl O-4-nitro-m-tolyl phosphorothioatehttps://eur-lex.europa.eu/eli/reg/2008/1272/oj
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/licenseO,O-DIMETHYL O-(3-METHYL-4-NITROPHENYL) PHOSPHOROTHIOATEhttps://mona.fiehnlab.ucdavis.edu/spectra/browse?query=exists(compound.metaData.name:%27InChIKey%27%20and%20compound.metaData.value:%27ZNOLGFHPUIJIMJ-UHFFFAOYSA-N%27)
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawFenitrothionhttp://www.nist.gov/srd/nist1a.cfm
- SpectraBaseFenitrothionhttps://spectrabase.com/spectrum/EoENmWsx0vbFenitrothionhttps://spectrabase.com/spectrum/5Yxj3RbOBl2phosphorothioic acid, O,O-dimethyl O-4-nitro-m-tolyl esterhttps://spectrabase.com/spectrum/3to2Jji5cLkFENITROTHION;O,O-DIEMTHYL-O-(3-METHYL-4-NITROPHENYL)-PHOSPHOROTHIOATEhttps://spectrabase.com/spectrum/JBgzOXAqJWIFENITROTHION;O,O-DIEMTHYL-O-(3-METHYL-4-NITROPHENYL)-PHOSPHOROTHIOATEhttps://spectrabase.com/spectrum/6fUhtM0nmjyPHOSPHOROTHIOIC ACID, O,O-DIMETHYL O-4-NITRO-M-TOLYL ESTERhttps://spectrabase.com/spectrum/3t1741guSkmphosphorothioic acid, O,O-dimethyl O-4-nitro-m-tolyl esterhttps://spectrabase.com/spectrum/FdqP5K9HYHFPHOSPHOROTHIOIC ACID, O,O-DIMETHYL O-4-NITRO-M-TOLYL ESTERhttps://spectrabase.com/spectrum/7SaiMtDxOCh
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlRisk category of Japanese OTC drugshttp://www.genome.jp/kegg-bin/get_htext?br08312.kegClassification of Japanese OTC drugshttp://www.genome.jp/kegg-bin/get_htext?br08313.kegAnimal drugs in Japanhttp://www.genome.jp/kegg-bin/get_htext?br08331.keg
- MassBank EuropeO,O-DIMETHYL O-(3-METHYL-4-NITROPHENYL) PHOSPHOROTHIOATEhttps://massbank.eu/MassBank/Result.jsp?inchikey=ZNOLGFHPUIJIMJ-UHFFFAOYSA-N
- Metabolomics Workbench
- USGS Columbia Environmental Research CenterLICENSEhttps://www.usgs.gov/foia
- Springer Nature
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidatafenitrothionhttps://www.wikidata.org/wiki/Q417614
- WikipediaFusidic acidhttps://en.wikipedia.org/wiki/Fusidic_acidFenitrothionhttps://en.wikipedia.org/wiki/Fenitrothion
- Wiley
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlFenitrothionhttps://www.ncbi.nlm.nih.gov/mesh/68005278Insecticideshttps://www.ncbi.nlm.nih.gov/mesh/68007306Cholinesterase Inhibitorshttps://www.ncbi.nlm.nih.gov/mesh/68002800
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403380144https://pubchem.ncbi.nlm.nih.gov/substance/403380144