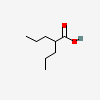
Valproic Acid
- VALPROIC ACID
- 2-Propylpentanoic acid
- 99-66-1
- 2-Propylvaleric acid
- Dipropylacetic acid
- Create:2005-03-25
- Modify:2025-01-18
 Valproate Sodium (has salt form);
Valproate Sodium (has salt form);  Divalproex Sodium (active moiety of);
Divalproex Sodium (active moiety of);  Magnesium Valproate (has salt form) ... View More ...
Magnesium Valproate (has salt form) ... View More ...
- 2 Propylpentanoic Acid
- 2-Propylpentanoic Acid
- Calcium Valproate
- Convulsofin
- Depakene
- Depakine
- Depakote
- Dipropyl Acetate
- Divalproex
- Divalproex Sodium
- Ergenyl
- Magnesium Valproate
- Propylisopropylacetic Acid
- Semisodium Valproate
- Sodium Valproate
- Valproate
- Valproate Calcium
- Valproate Sodium
- Valproic Acid
- Valproic Acid, Sodium Salt (2:1)
- Vupral
- VALPROIC ACID
- 2-Propylpentanoic acid
- 99-66-1
- 2-Propylvaleric acid
- Dipropylacetic acid
- Depakene
- Valproate
- Depakine
- Mylproin
- Ergenyl
- Di-n-propylacetic acid
- Propylvaleric acid
- Pentanoic acid, 2-propyl-
- n-Dipropylacetic acid
- 4-Heptanecarboxylic acid
- Myproic Acid
- Depakin
- n-DPA
- Acido valproico
- Acide valproique
- Di-n-propylessigsaure
- Dipropylacetate
- Convulex
- Stavzor
- Kyselina 2-propylvalerova
- Acidum valproicum
- 2-n-Propyl-n-valeric acid
- Acetic acid, dipropyl-
- Valproinsaeure
- Avugane
- Baceca
- 2,2-di-n-propylacetic acid
- Abbott 44090
- 2-PROPYL-PENTANOIC ACID
- Valeric acid, 2-propyl-
- Savicol
- Depakine chrono
- Depakin chrono
- Di-n-propylessigsaeure
- Acide valproique [INN-French]
- Acido valproico [INN-Spanish]
- Acidum valproicum [INN-Latin]
- Di-n-propylessigsaure [German]
- Epilim
- Kyselina 2-propylvalerova [Czech]
- VPA
- NSC 93819
- HSDB 3582
- pentanoic acid, 2-propyl
- EINECS 202-777-3
- VPA;2-Propylpentanoic Acid
- UNII-614OI1Z5WI
- MFCD00002672
- Valproic acid extended release
- BRN 1750447
- PEAC
- 614OI1Z5WI
- DTXSID6023733
- CHEBI:39867
- AI3-10500
- 2 Propylpentanoic Acid
- Depakene (TN)
- Vupral
- CHEMBL109
- (n-C3H7)2CHCOOH
- DTXCID803733
- EC 202-777-3
- NSC93819
- Valerin
- NSC-93819
- Valproic acid [USAN:USP:INN:BAN]
- NCGC00091149-01
- Deproic
- Alti-Valproic
- Novo-Valproic
- Penta-Valproic
- Dom-Valproic
- Med Valproic
- Nu-Valproic
- Valproic acid USP
- PMS-Valproic Acid
- VALPROIC ACID (MART.)
- VALPROIC ACID [MART.]
- Acide valproique (INN-French)
- Acido valproico (INN-Spanish)
- Acidum valproicum (INN-Latin)
- VALPROIC ACID (USP-RS)
- VALPROIC ACID [USP-RS]
- Valproic acid (USP)
- VALPROIC ACID (EP IMPURITY)
- VALPROIC ACID [EP IMPURITY]
- VALPROIC ACID (EP MONOGRAPH)
- VALPROIC ACID (USP IMPURITY)
- VALPROIC ACID [EP MONOGRAPH]
- VALPROIC ACID [USP IMPURITY]
- Valproic acid (USAN:USP:INN:BAN)
- VALPROIC ACID (USP MONOGRAPH)
- VALPROIC ACID [USP MONOGRAPH]
- CAS-99-66-1
- Depakote (TM)
- SMR000499581
- VALPROICACID
- 2 PP (base)
- Valproinsaure
- Valproic
- valproic-acid
- Novo-divalproex
- Sandoz valproic
- Dom-valproate
- Gen-divalproex
- Apo-valproic
- APO-divalproex
- DOM-divalproex
- Epival er
- PHL-valproate
- PMS-valproate
- PMS-Divalproex
- Erganyl; Stavzor
- Dom-valproic acid
- PHL-valproic acid
- Epiject I.V.
- 2-propyl-Pentanoate
- Epical (TM)
- Epilim (Salt/Mix)
- Depacon (Salt/Mix)
- Convulex (Salt/Mix)
- Eurekene (Salt/Mix)
- G2M-777
- Valparin (Salt/Mix)
- Valproic acid (GMP)
- Novo-Valproic - ECC
- Spectrum_000521
- Divalproex (Salt/Mix)
- Ratio-Valproic - ECC
- Valdisoval (Salt/Mix)
- 2 -propylpentanoic acid
- di-n-propyl acetic acid
- S(-)-4-En-valproate
- Spectrum2_000946
- Spectrum3_001733
- Spectrum4_000376
- Valproic acid (Standard)
- DOM-valproic acid E.C.
- PHL-valproic acid E.C.
- PMS-valproic acid E.C.
- Acidum valproicum (Latin)
- Novo-valproic soft gel cap
- SCHEMBL2275
- VALPROIC ACID [MI]
- VALPROIC ACID [INN]
- S-2-n-Propyl-4-pentenoate
- (S)-2-propyl-4-pentanoate
- KBioGR_000871
- KBioGR_002277
- KBioSS_001001
- KBioSS_002278
- VALPROIC ACID [HSDB]
- VALPROIC ACID [USAN]
- MLS001076682
- MLS001335927
- MLS001335928
- MLS002415770
- BIDD:GT0858
- DivK1c_000273
- VALPROIC ACID [VANDF]
- SPBio_000912
- GTPL7009
- VALPROIC ACID [WHO-DD]
- WLN: QVY3 & 3
- KBio1_000273
- KBio2_001001
- KBio2_002277
- KBio2_003569
- KBio2_004845
- KBio2_006137
- KBio2_007413
- KBio3_002626
- KBio3_002757
- NIJJYAXOARWZEE-UHFFFAOYSA-
- Valproic acid [USAN:BAN:INN]
- NINDS_000273
- GLXC-02555
- HMS2089J06
- HMS2231E06
- HMS3259C18
- HMS3370C21
- HMS3715B15
- HMS3885G14
- Valproic acid, 1mg/ml in Methanol
- ALBB-032973
- BCP33204
- VALPROIC ACID [ORANGE BOOK]
- Tox21_111091
- Tox21_201963
- Tox21_300603
- BDBM50003616
- HY-10585G
- HY-10585R
- LMFA01020291
- s3944
- STL445581
- AKOS009156895
- Tox21_111091_1
- Valproic Acid 1.0 mg/ml in Methanol
- CCG-221127
- CS-1765
- DB00313
- NC00584
- SDCCGSBI-0050864.P004
- SDCCGSBI-0050864.P012
- NCGC00091149-02
- NCGC00091149-03
- NCGC00091149-04
- NCGC00091149-05
- NCGC00091149-06
- NCGC00091149-08
- NCGC00091149-09
- NCGC00091149-21
- NCGC00091149-26
- NCGC00162288-07
- NCGC00254365-01
- NCGC00259512-01
- AS-11354
- DA-78822
- HY-10585
- SBI-0050864.P003
- NS00010277
- P0823
- EN300-64925
- C07185
- D00399
- AB00698315-06
- Q240642
- Q-200321
- SR-01000075242-7
- BRD-K41260949-001-18-2
- BRD-K41260949-236-15-0
- BRD-K41260949-236-16-8
- BRD-K41260949-236-17-6
- BRD-K41260949-236-18-4
- BRD-K41260949-236-19-2
- BRD-K41260949-236-20-0
- F2191-0115
- Z756391526
- Valproic acid, European Pharmacopoeia (EP) Reference Standard
- Valproic acid, United States Pharmacopeia (USP) Reference Standard
- InChI=1/C8H16O2/c1-3-5-7(6-4-2)8(9)10/h7H,3-6H2,1-2H3,(H,9,10)
- Valproic acid for system suitability, European Pharmacopoeia (EP) Reference Standard
- Valproic acid, Pharmaceutical Secondary Standard; Certified Reference Material
126.73 Ų [M+H]+ [CCS Type: TW; Method: calibrated with polyalanine and drug standards]
130.67 Ų [M+Na]+ [CCS Type: TW; Method: calibrated with polyalanine and drug standards]
71.04924 100
43.05433 79.70
145.12223 63.50
117.09079 41.10
69.07008 30.70
143.3 999
96.8 11
70.8 6
Valproate Sodium (has salt form)
Divalproex Sodium (active moiety of)
Magnesium Valproate (has salt form)
Valproate bismuth (is active moiety of)
- Brain
- Liver
- Extracellular
- Membrane
Use (kg) in Switzerland (2009): >7500
Use (kg; approx.) in Germany (2009): >75000
Use (kg; exact) in Germany (2009): 94896
Use (kg) in USA (2002): 229000
Use (kg) in France (2004): 112162
Consumption (g per capita) in Switzerland (2009): 0.96
Consumption (g per capita; approx.) in Germany (2009): 0.92
Consumption (g per capita; exact) in Germany (2009): 1.2
Consumption (g per capita) in the USA (2002): 0.81
Consumption (g per capita) in France (2004): 1.9
Excretion rate: 0.02
Calculated removal (%): 93.6



H302 (100%): Harmful if swallowed [Warning Acute toxicity, oral]
H315 (92.9%): Causes skin irritation [Warning Skin corrosion/irritation]
H318 (12.7%): Causes serious eye damage [Danger Serious eye damage/eye irritation]
H319 (80.2%): Causes serious eye irritation [Warning Serious eye damage/eye irritation]
H335 (31.7%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation]
H360 (64.3%): May damage fertility or the unborn child [Danger Reproductive toxicity]
P203, P261, P264, P264+P265, P270, P271, P280, P301+P317, P302+P352, P304+P340, P305+P351+P338, P305+P354+P338, P317, P318, P319, P321, P330, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 126 reports by companies from 17 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Acute Tox. 4 (100%)
Skin Irrit. 2 (92.9%)
Eye Dam. 1 (12.7%)
Eye Irrit. 2 (80.2%)
STOT SE 3 (31.7%)
Repr. 1A (64.3%)
Acute toxicity (Oral) - Category 4
Reproductive toxicity - Category 1A, Additional category: Effects on or via lactation
Specific target organ toxicity - Single exposure - Category 1 (central nervous system), Category 3 (narcotic effects)
Specific target organ toxicity - Repeated exposure - Category 1 (central nervous system, haemal system, liver)
SYMPTOMS: Symptoms of exposure to this compound may include gastrointestinal disturbances, hair loss, psychosis, altered bleeding time, altered liver enzymes and fatal hepatic failure. Other symptoms may include central nervous system depression, nausea, vomiting, indigestion, diarrhea, abdominal cramps, constipation, anorexia with weight loss, increased appetite with weight gain, tremor, ataxia, headache, nystagmus, diplopia, asterixis, spots before the eyes, dysarthria, dizziness, incoordination, coma, skin rash, erythema multiforme, generalized pruritus, emotional upset, depression, hyperactivity, behavioral deterioration, weakness, thrombocytopenia, petechiae, bruising, hematoma formation, frank hemorrhage, relative lymphocytosis, hypofibrinogenemia, leukopenia, eosinophilia, anemia, bone marrow suppression, irregular menses, secondary amenorrhea and breast enlargement. Changes in exocrine pancreas and sleep disturbances may also occur. It may also cause somnolence.
ACUTE/CHRONIC HAZARDS: When heated to decomposition this compound may emit toxic fumes of carbon monoxide and carbon dioxide. (NTP, 1992)
EYES: First check the victim for contact lenses and remove if present. Flush victim's eyes with water or normal saline solution for 20 to 30 minutes while simultaneously calling a hospital or poison control center. Do not put any ointments, oils, or medication in the victim's eyes without specific instructions from a physician. IMMEDIATELY transport the victim after flushing eyes to a hospital even if no symptoms (such as redness or irritation) develop.
SKIN: IMMEDIATELY flood affected skin with water while removing and isolating all contaminated clothing. Gently wash all affected skin areas thoroughly with soap and water. If symptoms such as redness or irritation develop, IMMEDIATELY call a physician and be prepared to transport the victim to a hospital for treatment.
INHALATION: IMMEDIATELY leave the contaminated area; take deep breaths of fresh air. If symptoms (such as wheezing, coughing, shortness of breath, or burning in the mouth, throat, or chest) develop, call a physician and be prepared to transport the victim to a hospital. Provide proper respiratory protection to rescuers entering an unknown atmosphere. Whenever possible, Self-Contained Breathing Apparatus (SCBA) should be used; if not available, use a level of protection greater than or equal to that advised under Protective Clothing.
INGESTION: DO NOT INDUCE VOMITING. If the victim is conscious and not convulsing, give 1 or 2 glasses of water to dilute the chemical and IMMEDIATELY call a hospital or poison control center. Be prepared to transport the victim to a hospital if advised by a physician. If the victim is convulsing or unconscious, do not give anything by mouth, ensure that the victim's airway is open and lay the victim on his/her side with the head lower than the body. DO NOT INDUCE VOMITING. IMMEDIATELY transport the victim to a hospital. (NTP, 1992)
SMALL SPILLS AND LEAKAGE: If you spill this chemical, FIRST REMOVE ALL SOURCES OF IGNITION. Then, use absorbent paper to pick up all liquid spill material. Your contaminated clothing and absorbent paper should be sealed in a vapor-tight plastic bag for eventual disposal. Solvent wash all contaminated surfaces with 60-70% ethanol followed by washing with a soap and water solution. Do not reenter the contaminated area until the Safety Officer (or other responsible person) has verified that the area has been properly cleaned.
STORAGE PRECAUTIONS: You should store this chemical under ambient temperatures, and keep it away from oxidizing materials. (NTP, 1992)
Hazard Traits - Developmental Toxicity
Authoritative List - Prop 65
Report - regardless of intended function of ingredient in the product
Status: Active Update: 11-04-2019 https://echa.europa.eu/registration-dossier/-/registered-dossier/14340
Status: Cease Manufacture Update: 03-10-2011 https://echa.europa.eu/registration-dossier/-/registered-dossier/7482
Prospective studies suggest that 5% to 10% of persons develop ALT elevations during long term valproate therapy, but these abnormalities are usually asymptomatic and can resolve even with continuation of drug. Unlike phenytoin and carbamazepine, valproate does not induce elevations in serum GGT levels. More importantly and not uncommonly, valproate can cause several forms of clinically apparent hepatotoxicity. Indeed, more than 100 fatal cases of acute or chronic liver injury due to valproate have been reported in the literature. Three clinically distinguishable forms of hepatotoxicity (besides simple aminotransferase elevations) can occur with valproate.
The first syndrome is hyperammonemia with minimal or no evidence of hepatic injury. This syndrome typically presents with progressive and episodic confusion followed by obtundation and coma. The time to onset is often within a few weeks of starting valproate or increasing the dose, but it can present months or even years after starting the medication (Case 1). The diagnosis is made by the finding of elevations in serum ammonia with normal (or near normal) serum aminotransferase and bilirubin levels. Valproate levels are usually normal or minimally high. The syndrome resolves within a few days of stopping valproate, but may reverse more rapidly with carnitine supplementation or renal hemodialysis.
The second form of injury from valproate is an acute hepatocellular injury with jaundice, typically accompanied by hepatocellular or mixed pattern of enzyme elevations (Case 2). This acute liver injury pattern usually has its onset within 1 to 6 months of starting valproate. The pattern of serum enzyme elevations can be hepatocellular or mixed; sometimes the serum aminotransferase levels are not markedly elevated, despite the severity of injury.
Immunoallergic features (fever, rash, eosinophilia) are usually absent, but rare cases with prominent features of hypersensitivity have been reported (Case 3). Multiple instances of fatal acute hepatic failure due to valproate have been published and valproate is regularly listed as a cause of drug induced acute liver failure. Liver histology is distinctive and reveals a microvesicular steatosis with central lobular necrosis, mild to moderate inflammation and cholestasis. In cases with a prolonged course, fibrosis, bile duct proliferation and regenerative nodules may be present. Prospective studies using historical controls suggest that carnitine (particularly intravenously) may be beneficial if given soon after presentation.
The third form of hepatic injury due to valproate is a Reye-like syndrome described in children on valproate who develop fever and lethargy (suggestive of a viral infection) followed by confusion, stupor and coma, with raised ammonia levels and marked ALT elevations but normal or minimally elevated bilirubin levels. Metabolic acidosis is also common and the syndrome can be rapidly fatal. Valproate may simply be an aspirin-like agent capable of triggering Reye syndrome if it is being taken when the child develops either influenza or varicella infection.
All three forms of valproate hepatotoxicity have features of mitochondrial injury, and liver histology usually demonstrates microvesicular steatosis with variable amounts of inflammation and cholestasis. Young age (
Likelihood score: A (well known cause of several forms of clinically apparent liver injury).
M Chen, V Vijay, Q Shi, Z Liu, H Fang, W Tong. FDA-Approved Drug Labeling for the Study of Drug-Induced Liver Injury, Drug Discovery Today, 16(15-16):697-703, 2011. PMID:21624500 DOI:10.1016/j.drudis.2011.05.007
M Chen, A Suzuki, S Thakkar, K Yu, C Hu, W Tong. DILIrank: the largest reference drug list ranked by the risk for developing drug-induced liver injury in humans. Drug Discov Today 2016, 21(4): 648-653. PMID:26948801 DOI:10.1016/j.drudis.2016.02.015
◉ Summary of Use during Lactation
Valproic acid levels in breastmilk are low and infant serum levels range from undetectable to low. Breastfeeding during valproic acid monotherapy does not appear to adversely affect infant growth or development; however, and breastfed infants had higher IQs and enhanced verbal abilities than nonbreastfed infants at 6 years of age in one study. A safety scoring system finds valproic acid possible to use during breastfeeding, and a computer model predicted a relatively low infant exposure, consistent with literature reports. If valproic acid is required by the mother, it is not a reason to discontinue breastfeeding.
No definite adverse reactions to valproic acid in breastfed infants have been reported. Theoretically, breastfed infants are at risk for valproic acid-induced hepatotoxicity, so infants should be monitored for jaundice and other signs of liver damage during maternal therapy. A questionable case of thrombocytopenia has been reported, so monitor the infant for unusual bruising or bleeding. A rare case of infant baldness might have been caused by valproate in milk. Observe the infant for jaundice and unusual bruising or bleeding. Combination therapy with sedating anticonvulsants or psychotropics may result in infant sedation or withdrawal reactions.
◉ Effects in Breastfed Infants
A mother with epilepsy was taking valproic acid 2.4 grams daily and primidone 250 mg 3 times daily during pregnancy and postpartum. During the second week postpartum, her breastfed infant was sedated. Breastfeeding was stopped and the drowsiness cleared. The sedation was possibly caused by primidone in breastmilk although valproic acid might have contributed by increasing primidone levels.
Petechiae, thrombocytopenia, anemia, and mild hematuria occurred in a 2.5-month-old breastfed infant whose mother was taking valproic acid 600 mg twice daily. Blood hemoglobin and reticulocytes normalized between 12 and 19 days after discontinuing breastfeeding. The petechiae resolved 35 days after discontinuing breastfeeding and the infant's platelet count had almost reached the normal range by this time. By day 83, the infant's platelet count was well within the normal range. The authors believed the adverse effect to be caused by valproic acid in breastmilk. However, other authors believe that these symptoms were more likely caused by idiopathic thrombocytopenic purpura following a viral infection.
Two breastfed infants aged 1 and 3 months whose mothers were taking valproic acid monotherapy 750 and 500 mg daily developed normally and had no abnormal laboratory values. Their plasma levels were 6% and 1.5% or their mother's serum levels, respectively.
Six breastfed infants whose mothers were taking valproic acid 750 or 1000 mg daily had no adverse reactions to valproic acid in breastmilk.
An exclusively breastfed infants whose mother was taking valproate 1.8 g, topiramate 300 mg, and levetiracetam 2 g, daily during pregnancy and lactation appeared healthy to the investigators throughout the 6- to 8-week study period.
In a long-term study on infants exposed to anticonvulsants during breastfeeding, no difference in average intelligence quotient at 3 years of age was found between infants who were breastfed (n = 11) a median of 6 months and those not breastfed (n = 24) when their mothers were taking valproate monotherapy. At 6 years of age, extensive psychological and intelligence testing found that the breastfed infants had higher IQ values than the nonbreastfed infants.
A prospective cohort study in Norway followed infants of mothers who took antiepileptic drugs during pregnancy and lactation and compared them to infants of mothers with untreated epilepsy and infants with fathers who took antiepileptics as control groups. Of the 223 mothers studied, 27 were taking valproate monotherapy. Infants were assessed at 6, 18 and 36 months of age. Continuous breastfeeding in children of women using antiepileptic drugs was associated with no greater impaired development than those with no breastfeeding or breastfeeding for less than 6 months.
A woman with bipolar disorder who delivered twins and was taking sodium valproate in a therapeutic dosage was started on quetiapine 200 mg and olanzapine 15 mg at 11 pm daily after 20 days postpartum. She withheld breastfeeding during the night and discarded milk pumped at 7 am. She then breastfed her infants until 11 pm. The mother continued feeding the infants on this schedule for 15 months. Monthly follow-up of the infants indicated normal growth and neither the pediatricians nor the parents noted any adverse effects in the infants.
The 4-month-old breastfed infant of a mother taking divalproex for bipolar disorder developed patchy hair loss. The extent of nursing and dosage of divalproex were not stated. Divalproex was discontinued and 2 months later, the infant’s hair was normal. The hair loss was possibly caused by valproate.
◉ Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
◉ Summary of Use during Lactation
Very little information is available on the clinical use of divalproex during breastfeeding. However, divalproex is rapidly metabolized in the body to the active drug valproic acid. Valproic acid levels in breastmilk are low and infant serum levels range from undetectable to low. Breastfeeding during valproic acid monotherapy does not appear to adversely affect infant growth or development, and breastfed infants had higher IQs and enhanced verbal abilities than nonbreastfed infants at 6 years of age in one study. A safety scoring system finds valproic acid possible to use during breastfeeding. If valproic acid is required by the mother, it is not necessarily a reason to discontinue breastfeeding.
No definite adverse reactions to valproic acid in breastfed infants have been reported. Theoretically, breastfed infants are at risk for valproic acid-induced hepatotoxicity, so infants should be monitored for jaundice and other signs of liver damage during maternal therapy. A questionable case of thrombocytopenia has been reported, so monitor the infant for unusual bruising or bleeding. A rare case of infant baldness might have been caused by valproate in milk. Observe the infant for jaundice and unusual bruising or bleeding. Combination therapy with sedating anticonvulsants or psychotropics may result in infant sedation or withdrawal reactions.
◉ Effects in Breastfed Infants
A mother with epilepsy was taking valproic acid 2.4 grams daily and primidone 250 mg 3 times daily during pregnancy and postpartum. During the second week postpartum, her breastfed infant was sedated. Breastfeeding was stopped and the drowsiness cleared. The sedation was possibly caused by primidone in breastmilk although valproic acid might have contributed by increasing primidone levels.
Petechiae, thrombocytopenia, anemia, and mild hematuria occurred in a 2.5-month-old breastfed infant whose mother was taking valproic acid 600 mg twice daily. Blood hemoglobin and reticulocytes normalized between 12 and 19 days after discontinuing breastfeeding. The petechiae resolved 35 days after discontinuing breastfeeding and the infant's platelet count had almost reached the normal range by this time. By day 83, the infant's platelet count was well within the normal range. The authors believed the adverse effect to be caused by valproic acid in breastmilk. However, other authors believe that these symptoms were more likely caused by idiopathic thrombocytopenic purpura following a viral infection.
Two breastfed infants aged 1 and 3 months whose mothers were taking valproic acid monotherapy 750 and 500 mg daily developed normally and had no abnormal laboratory values. Their plasma levels were 6% and 1.5% or their mother's serum levels, respectively.
Six breastfed infants whose mothers were taking valproic acid 750 or 1000 mg daily had no adverse reactions to valproic acid in breastmilk.
An exclusively breastfed infants whose mother was taking valproate 1.8 g, topiramate 300 mg, and levetiracetam 2 g, daily during pregnancy and lactation appeared healthy to the investigators throughout the 6- to 8-week study period.
In a long-term study on infants exposed to anticonvulsants during breastfeeding, no difference in average intelligence quotient at 3 years of age was found between infants who were breastfed (n = 11) a median of 6 months and those not breastfed (n = 24) when their mothers were taking valproate monotherapy. At 6 years of age, extensive psychological and intelligence testing found that the breastfed infants had higher IQ values than the nonbreastfed infants.
A prospective cohort study in Norway followed infants of mothers who took antiepileptic drugs during pregnancy and lactation and compared them to infants of mothers with untreated epilepsy and infants with fathers who took antiepileptics as control groups. Of the 223 mothers studied, 27 were taking valproate monotherapy. Infants were assessed at 6, 18 and 36 months of age. Continuous breastfeeding in children of women using antiepileptic drugs was associated with no greater impaired development than those with no breastfeeding or breastfeeding for less than 6 months.
A woman with bipolar disorder who delivered twins and was taking sodium valproate in a therapeutic dosage was started on quetiapine 200 mg and olanzapine 15 mg at 11 pm daily after 20 days postpartum. She withheld breastfeeding during the night and discarded milk pumped at 7 am. She then breastfed her infants until 11 pm. The mother continued feeding the infants on this schedule for 15 months. Monthly follow-up of the infants indicated normal growth and neither the pediatricians nor the parents noted any adverse effects in the infants.
The 4-month-old breastfed infant of a mother taking divalproex for bipolar disorder developed patchy hair loss. The extent of nursing and dosage of divalproex were not stated. Divalproex was discontinued and 2 months later, the infant’s hair was normal. The hair loss was possibly caused by valproate.
◉ Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
◈ What is valproic acid?
Valproic acid is a medication that has been used to control seizures in the treatment of epilepsy, and to treat bipolar disorder and migraines. Valproic acid is sometimes also called sodium valproate or valproate sodium. Some brand names for valproic acid are Depakene®, Stavzor®, and Depacon®. A similar medication, divalproex (Depakote®), breaks down into valproic acid in the body.The Food and Drug Administration recommends that people who are pregnant do not take valproate sodium and related products, valproic acid and divalproex sodium to prevent migraine headaches. For epilepsy or bipolar disorder, valproate products should only be prescribed during pregnancy if other medications are not effective in treating the condition or cannot be used for another reason.Sometimes when people find out they are pregnant, they think about changing how they take their medication, or stopping their medication altogether. However, it is important to talk with your healthcare providers before making any changes to how you take your medication. Your healthcare providers can talk with you about the benefits of treating your condition and the risks of untreated illness during pregnancy.
◈ I am taking valproic acid, but I would like to stop taking it before getting pregnant. How long does the drug stay in my body?
People eliminate medications at different rates. In healthy adults, it takes 2-4 days, on average, for most of the valproic acid to be gone from the body.
◈ What might happen if I stopped taking my valproic acid and then had a seizure during my pregnancy?
Having a seizure while pregnant might be harmful to the fetus. Complications depend on many things, such as the type of seizure, how long the seizure lasts, and the number of seizures that happen. Epileptic seizures might cause periods of time when the fetus is not getting enough oxygen, which could lead to problems with development. These seizures could also be life-threatening for both the person who is pregnant and the fetus. A seizure could cause a person who is pregnant to fall or have an accident that could injure themselves or the fetus.
◈ What might happen if I stopped taking my valproic acid and then had a relapse of bipolar disorder during my pregnancy?
People who are pregnant and have bipolar disorder who stop taking their medication during pregnancy might have a higher chance for symptoms of depression or mania that could be harmful to both the person who is pregnant and the fetus. Episodes of depression or mania are very stressful for a person who is pregnant. During manic or depressive episodes, the person who is pregnant might have more trouble taking care of themselves and keeping themselves safe.
◈ I take valproic acid. Can it make it harder for me to get pregnant?
Some studies suggest that people on valproic acid might have a higher chance of developing polycystic ovary syndrome (PCOS), a condition associated with trouble getting pregnant. Studies have found that people with seizure disorders and people with bipolar disorder might have problems with their periods and trouble getting pregnant. This possible increase might be due to the conditions that the people have, rather than the use of medication.
◈ Does taking valproic acid increase the chance of miscarriage?
Miscarriage is common and can occur in any pregnancy for many different reasons. It is not known if valproic acid increases the chance of miscarriage.
◈ Does taking valproic acid increase the chance of birth defects?
Every pregnancy starts out with a 3-5% chance of having a birth defect. This is called the background risk. Studies have found that taking valproic acid in pregnancy is associated with a chance of having a baby with fetal valproate spectrum disorder which includes minor and major birth defects. Birth defects are typically classified as major if they need surgery to be repaired. Some of the birth defects that are more likely to happen include heart defects, cleft lip (when the lip does not form correctly and needs surgery to repair after birth), or neural tube defects (an opening in the baby’s spine or skull). Some babies exposed to valproic acid might also have more minor birth defects like facial differences, such as a thin upper lip. The chance of a birth defect seems to be greater with higher doses of valproic acid or with taking it with another seizure medication.The most common neural tube defect linked to valproic acid use is spina bifida (opening in the spine). The chance of a neural tube defect when taking valproic acid is approximately 1 in 50 to 1 in 100 (1-2%). Taking extra folic acid before trying to get pregnant and in early pregnancy might help lower the chance of some birth defects in pregnancies exposed to valproic acid. Talk to your healthcare provider about how much folic acid you should take. For more information on folic acid, please see the MotherToBaby fact sheet at: https://mothertobaby.org/fact-sheets/folic-acid/.
◈ Does taking valproic in pregnancy increase the chance of other pregnancy-related problems?
Valproic acid might increase the chance of low birth weight (weighing less than 5 pounds, 8 ounces [2500 grams] at birth). There have been reports of temporary low blood sugar levels (hypoglycemia) in newborns.
◈ Does taking valproic in pregnancy affect future behavior or learning for the child?
Prenatal exposure to valproic acid can increase the chance of problems with learning and development. Different studies have shown an increased chance of intellectual disability, developmental delay, autism spectrum disorder, other developmental disorders, attention deficit/hyperactivity disorder, attachment disorder, decreased language and memory skills, and decreased social and adaptive behavior skills. Not all studies have shown the same results. Some of the long-term problems in the exposed children might be due to how severe the seizure disorder is in the person who is pregnant.
◈ What screenings or tests are available to see if my pregnancy has birth defects or other issues?
There are ways to screen for neural tube defects in pregnancy. A blood test can be done to measure the amount of a protein called alpha fetoprotein (AFP) in the blood of the person who is pregnant. Babies with spina bifida have higher levels of AFP. If the AFP is higher than usual in the blood test, more testing or screenings might be offered to you to get more information.An ultrasound that looks at the fetal spine can be used to screen for spina bifida. Ultrasounds can also screen for some other birth defects, such as a heart defect or cleft lip. Talk with your healthcare provider about any prenatal screenings or testing that are available to you. There are no tests available during pregnancy that can tell how much effect there could be on future behavior or learning.
◈ Breastfeeding while taking valproic acid:
The amount of valproic acid that passes into breast milk is low and blood levels from exposed infants are low to undetectable. There is a theoretical (not proven) concern that infants exposed to valproic acid through breastmilk could develop liver toxicity, so infants should be monitored for any changes or problems. If you suspect the baby has symptoms such as jaundice (yellowing of the skin or eyes), rash, or fever, contact the child’s healthcare provider. Be sure to talk to your healthcare provider about all your breastfeeding questions.
◈ If a male takes valproic acid, could it affect fertility or increase the chance of birth defects?
It is not known if valproic acid could affect male fertility (ability to get partner pregnant) or increase the chance of birth defects above the background risk. In general, exposures that sperm donors or fathers have are unlikely to increase risks to a pregnancy. For more information, please see the MotherToBaby fact sheet Paternal Exposures at https://mothertobaby.org/fact-sheets/paternal-exposures-pregnancy/.Available pregnancy registries include:US: North American Antiepileptic Drug (AED) Pregnancy Registry: 1-888-233-2334 or http://www.aedpregnancyregistry.org/Europe and other continents: the EURAP Registry (International Registry of Antiepileptic Drugs and Pregnancy) http://eurapinternational.org/index.Psychiatric medications: https://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/.
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=NIJJYAXOARWZEE-UHFFFAOYSA-N
- Avoid alcohol.
- Avoid milk and dairy products.
- Take with food.
- Australian Industrial Chemicals Introduction Scheme (AICIS)Pentanoic acid, 2-propyl-https://services.industrialchemicals.gov.au/search-assessments/Pentanoic acid, 2-propyl-https://services.industrialchemicals.gov.au/search-inventory/
- CAMEO ChemicalsLICENSECAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data.https://cameochemicals.noaa.gov/help/reference/terms_and_conditions.htm?d_f=falseVALPROIC ACIDhttps://cameochemicals.noaa.gov/chemical/21210CAMEO Chemical Reactivity Classificationhttps://cameochemicals.noaa.gov/browse/react
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusValproic acid [USAN:USP:INN:BAN]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000099661ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_useValproic acidhttps://www.drugbank.ca/drugs/DB00313
- DTP/NCILICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuse
- EPA DSSToxValproic acidhttps://comptox.epa.gov/dashboard/DTXSID6023733CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-notice2-propylvaleric acidhttps://chem.echa.europa.eu/100.002.5252-propylvaleric acid (EC: 202-777-3)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/54482
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Hazardous Substances Data Bank (HSDB)VALPROIC ACIDhttps://pubchem.ncbi.nlm.nih.gov/source/hsdb/3582
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingValproic acidhttp://www.hmdb.ca/metabolites/HMDB0001877HMDB0001877_nmr_one_1774https://hmdb.ca/metabolites/HMDB0001877#spectra
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/
- California Safe Cosmetics Program (CSCP) Product DatabaseValproate (Valproic acid)https://www.cdph.ca.gov/Programs/CCDPHP/DEODC/OHB/CSCP/Pages/About-CSCP.aspx
- BindingDBLICENSEAll data curated by BindingDB staff are provided under the Creative Commons Attribution 3.0 License (https://creativecommons.org/licenses/by/3.0/us/).https://www.bindingdb.org/rwd/bind/info.jsp2-n-propyl-n-valeric acidhttps://www.bindingdb.org/rwd/bind/chemsearch/marvin/MolStructure.jsp?monomerid=50003616
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jspValproic Acidhttps://ctdbase.org/detail.go?type=chem&acc=D014635
- Drug Gene Interaction database (DGIdb)LICENSEThe data used in DGIdb is all open access and where possible made available as raw data dumps in the downloads section.http://www.dgidb.org/downloadsVALPROIC ACIDhttps://www.dgidb.org/drugs/rxcui:11118
- IUPHAR/BPS Guide to PHARMACOLOGYLICENSEThe Guide to PHARMACOLOGY database is licensed under the Open Data Commons Open Database License (ODbL) https://opendatacommons.org/licenses/odbl/. Its contents are licensed under a Creative Commons Attribution-ShareAlike 4.0 International License (http://creativecommons.org/licenses/by-sa/4.0/)https://www.guidetopharmacology.org/about.jsp#licenseGuide to Pharmacology Target Classificationhttps://www.guidetopharmacology.org/targets.jsp
- Therapeutic Target Database (TTD)Valproic Acidhttps://idrblab.net/ttd/data/drug/details/D0KP0W
- Toxin and Toxin Target Database (T3DB)LICENSET3DB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (T3DB) and the original publication.http://www.t3db.ca/downloadsValproic acidhttp://www.t3db.ca/toxins/T3D2558
- California Office of Environmental Health Hazard Assessment (OEHHA)Valproate (Valproic acid)https://oehha.ca.gov/proposition-65/chemicals/valproate-valproic-acid
- ChEBI
- FDA Pharm ClassesLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingFDA Pharmacological Classificationhttps://www.fda.gov/ForIndustry/DataStandards/StructuredProductLabeling/ucm162549.htm
- LiverTox
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/Valproic acidhttps://www.wikidata.org/wiki/Q240642LOTUS Treehttps://lotus.naturalproducts.net/
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licenceVALPROIC ACIDhttps://platform.opentargets.org/drug/CHEMBL109
- CCSbaseCCSbase Classificationhttps://ccsbase.net/
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/VALPROIC ACIDNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- DailyMed
- Drug Induced Liver Injury Rank (DILIrank) DatasetLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Drugs and Lactation Database (LactMed)Valproic Acidhttps://www.ncbi.nlm.nih.gov/books/n/lactmed/LM403/
- Mother To Baby Fact SheetsLICENSECopyright by OTIS. This work is available under the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported license (CC BY-NC-ND 3.0).https://www.ncbi.nlm.nih.gov/books/about/copyright/
- Drugs@FDALICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingVALPROIC ACIDhttps://www.accessdata.fda.gov/scripts/cder/daf/
- EPA Chemical and Products Database (CPDat)EPA CPDat Classificationhttps://www.epa.gov/chemical-research/chemical-and-products-database-cpdat
- EU Clinical Trials Register
- NITE-CMC2-Propan-1-ylpentanoic acid [Valproic acid] - FY2017 (New/original classication)https://www.chem-info.nite.go.jp/chem/english/ghs/17-mhlw-0004e.html
- FDA Medication GuidesLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingDEPAKENEhttps://dps.fda.gov/medguide
- FDA Orange BookLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- National Drug Code (NDC) DirectoryLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- SpectraBase2-Propylpentanoic acidhttps://spectrabase.com/spectrum/2QdWaUlg6Dk2-Propylpentanoic acidhttps://spectrabase.com/spectrum/CaPHFC4LtgqValproic acidhttps://spectrabase.com/spectrum/Eb4eH4Yyj1uValproic acidhttps://spectrabase.com/spectrum/KInjBpHuHqQValproic acidhttps://spectrabase.com/spectrum/IdnWEq9bP3N2-Propylpentanoic acidhttps://spectrabase.com/spectrum/pSC5ZtftXK2-PROPYLVALERIC ACIDhttps://spectrabase.com/spectrum/EoiafeK4FIi2-Propylpentanoic acidhttps://spectrabase.com/spectrum/Hh8GgiHotlJ2-Propylpentanoic acidhttps://spectrabase.com/spectrum/HtO7FkgpFSv2-Propylpentanoic acidhttps://spectrabase.com/spectrum/7buaaqpHN96
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlUSP drug classificationhttp://www.genome.jp/kegg-bin/get_htext?br08302.kegAnatomical Therapeutic Chemical (ATC) classificationhttp://www.genome.jp/kegg-bin/get_htext?br08303.kegTarget-based classification of drugshttp://www.genome.jp/kegg-bin/get_htext?br08310.keg
- LIPID MAPSLipid Classificationhttps://www.lipidmaps.org/
- Natural Product Activity and Species Source (NPASS)
- MassBank Europe
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- Metabolomics Workbench
- Nature Chemical Biology
- NIPH Clinical Trials Search of Japan
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawValproic Acidhttp://www.nist.gov/srd/nist1a.cfm
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.htmlvalproic acidhttps://rxnav.nlm.nih.gov/id/rxnorm/11118
- NMRShiftDB
- WHO Anatomical Therapeutic Chemical (ATC) ClassificationLICENSEUse of all or parts of the material requires reference to the WHO Collaborating Centre for Drug Statistics Methodology. Copying and distribution for commercial purposes is not allowed. Changing or manipulating the material is not allowed.https://www.whocc.no/copyright_disclaimer/Valproic acidhttps://www.whocc.no/atc_ddd_index/?code=N03AG01
- PharmGKBLICENSEPharmGKB data are subject to the Creative Commons Attribution-ShareALike 4.0 license (https://creativecommons.org/licenses/by-sa/4.0/).https://www.pharmgkb.org/page/policiesvalproic acidhttps://www.pharmgkb.org/chemical/PA451846
- PharosLICENSEData accessed from Pharos and TCRD is publicly available from the primary sources listed above. Please respect their individual licenses regarding proper use and redistribution.https://pharos.nih.gov/aboutvalproic acidhttps://pharos.nih.gov/ligands/F3BQDP62S1D8
- Protein Data Bank in Europe (PDBe)
- Springer Nature
- SpringerMaterials2-Propylpentanoic acidhttps://materials.springer.com/substanceprofile/docs/smsid_bczzadnhoqcvtngs
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidatavalproic acidhttps://www.wikidata.org/wiki/Q240642
- Wikipedia
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlValproic Acidhttps://www.ncbi.nlm.nih.gov/mesh/68014635Enzyme Inhibitorshttps://www.ncbi.nlm.nih.gov/mesh/68004791Antimanic Agentshttps://www.ncbi.nlm.nih.gov/mesh/68018692Anticonvulsantshttps://www.ncbi.nlm.nih.gov/mesh/68000927GABA Agentshttps://www.ncbi.nlm.nih.gov/mesh/68018682
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 388324578https://pubchem.ncbi.nlm.nih.gov/substance/388324578
- NCBI