Valacyclovir
- valacyclovir
- Valaciclovir
- 124832-26-4
- ValACV
- Zelitrex
- Create:2019-01-15
- Modify:2025-01-18
 Acyclovir (has active moiety);
Acyclovir (has active moiety);  Valacyclovir Hydrochloride (has salt form);
Valacyclovir Hydrochloride (has salt form);  Valacyclovir hydrochloride monohydrate (active moiety of).
Valacyclovir hydrochloride monohydrate (active moiety of).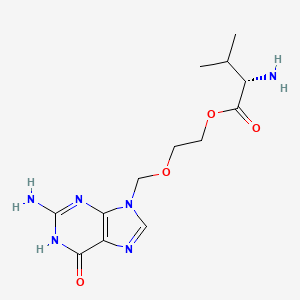
- 2-((2-amino-1,6-dihydro-6-oxo-9H-purin-9-yl)methoxy)ethyl L-valinate
- 256U87
- Acyclovir, L valyl Ester
- acyclovir, L-valyl ester
- BW256U87
- D- Valacyclovir
- L Valylacyclovir
- L-valyl Ester Acyclovir
- L-valylacyclovir
- valaciclovir
- valacyclovir
- valacyclovir hydrochloride
- valacyclovir hydrochloride, (DL)-isomer
- valacyclovir, (D)-isomer
- valacyclovir, (DL)-isomer
- valacyclovir, (L)-isomer
- Valacyclovir, D-
- valacyclovir, x-hydrochloride, (D)-isomer
- valacyclovir, x-hydrochloride, (DL)-isomer
- Valtrex
- valacyclovir
- Valaciclovir
- 124832-26-4
- ValACV
- Zelitrex
- Valcivir
- Valcyclovir
- Val-ACV
- L-Valine ester with 9-((2-hydroxyethoxy)methyl)guanine
- UNII-MZ1IW7Q79D
- MZ1IW7Q79D
- L-Valine, 2-((2-amino-1,6-dihydro-6-oxo-9H-purin-9-yl)methoxy)ethyl ester
- Valaciclovir (INN)
- CHEBI:35854
- HSDB 8084
- Valtrex (TN)
- CHEMBL1349
- 2-[(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methoxy]ethyl L-valinate
- 256U87 HCl
- DTXSID1023732
- (S)-2-[(2-Amino-6-oxo-6,9-dihydro-3H-purin-9-yl)methoxy]ethyl-2-amino-3-methylbutanoate
- BW 256U87
- MFCD01861507
- VALACICLOVIR [INN]
- 2-[(2-amino-6-oxo-1H-purin-9-yl)methoxy]ethyl (2S)-2-amino-3-methylbutanoate
- L-Valine, 2-[(2-amino-1,6-dihydro-6-oxo-9H-purin-9-yl)methoxy]ethyl ester
- 2-[(2-amino-6-oxo-6,9-dihydro-1H-purin-9-yl)methoxy]ethyl (2S)-2-amino-3-methylbutanoate
- Valaciclovir [INN:BAN]
- Talavir
- Virval
- L-VALINE, ESTER WITH 9-((2-HYDROXYETHOXY)METHYL)GUANINE
- Valacyclover Hydrochloric
- Acyclovir-valine
- (S)-2-((2-Amino-6-oxo-1H-purin-9(6H)-yl)methoxy)ethyl 2-amino-3-methylbutanoate
- 2-[(2-amino-6-oxo-6,9-dihydro-3H-purin-9-yl)methoxy]ethyl (2S)-2-amino-3-methylbutanoate
- VACV
- Valaciclovir, Valtrex
- BW256U87
- NCGC00159520-02
- Valaciclovirum
- 2-((2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methoxy)ethyl L-valinate
- BW-256U
- TXC
- Valtrex (TM)
- Valcyclovir [INN:BAN]
- L-Valine ester with 9-
- VALACYCLOVIR [MI]
- 2-[(2-amino-6-oxo-3H-purin-9-yl)methoxy]ethyl (2S)-2-amino-3-methylbutanoate
- 256U87
- VALACYCLOVIR [VANDF]
- SCHEMBL28644
- BSPBio_002474
- MLS001304747
- MLS006011776
- VALACICLOVIR [WHO-DD]
- DTXCID403732
- GTPL4824
- J05AB11
- HMS2090D20
- HMS2234I15
- BCP28415
- BBL033701
- BDBM50162073
- STK802272
- STL535579
- ZB0748
- 2-{[(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methyl]oxy}ethyl L-valinate
- AKOS005622706
- AKOS007930693
- AKOS025149468
- AKOS037496964
- CS-1356
- DB00577
- valacyclovir hydrochloride (monohydrate)
- NCGC00178638-04
- NCGC00178638-05
- NCGC00178638-15
- Valaciclovir;Val-ACV; Valtrex; Zelitrex
- AC-11128
- HY-17425
- SMR000752514
- SMR004703478
- SBI-0206711.P001
- NS00009305
- D08664
- EN300-150220
- G78316
- AB00698497-11
- AB01275525-01
- AB01275525_02
- AB01275525_03
- Q418594
- W-200980
- BRD-K46435977-003-01-2
- BRD-K46435977-003-03-8
- BRD-K46435977-003-11-1
- BRD-K46435977-003-12-9
- 2-[(2-amino-6-hydroxy-9H-purin-9-yl)methoxy]ethyl L-valinate
- 2-[(2-amino-1,6-dihydro-6-oxo-9h-purin-9-yl)methoxy]ethyl-l-valinate
- (S)-2-((2-amino-6-oxo-3H-purin-9(6H)-yl)methoxy)ethyl 2-amino-3-methylbutanoate
- 2-[(2-amino-6-oxo-1H-purin-9-yl)methoxy]ethyl (2S)-2-amino-3-methyl-butanoate
- L-VALINE 2-((2-AMINO-1,6-DIHYDRO-6-OXO-9H-PURIN-9-YL)METHOXY)ETHYL ESTER
- (S)-2-Amino-3-methyl-butyric acid 2-(2-amino-6-oxo-1,6-dihydro-purin-9-ylmethoxy)-ethyl ester
164.19 Ų [M+H-H2O]+ [CCS Type: TW; Method: calibrated with polyalanine and drug standards]
166.89 Ų [M+H]+ [CCS Type: TW; Method: calibrated with polyalanine and drug standards]
152.055618 100
135.027756 37.83
146.116974 15.29
144.103165 7.77
67.901878 7.64
152.0562 100
72.08053 17.74
135.0295 10.59
84.08189 7.78
146.11731 6.32
116.07042 100
176.05684 25.82
323.14694 21.14
206.06767 14.54
162.04108 3.88
152.0562 100
72.08053 17.74
135.0295 10.59
84.08189 7.78
146.11731 6.32
Acyclovir (has active moiety)
Valacyclovir Hydrochloride (has salt form)
Valacyclovir hydrochloride monohydrate (active moiety of)
- Cytoplasm
- Membrane
Use (kg; approx.) in Germany (2009): >750
Use (kg) in USA (2002): 63500
Consumption (g per capita; approx.) in Germany (2009): 0.00916
Consumption (g per capita) in the USA (2002): 0.225
Excretion rate: 0.01
Calculated removal (%): 22

P264, P270, P301+P317, P330, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Oral therapy with valacyclovir is associated with a low rate of mild-to-moderate serum aminotransferase elevations, but these abnormalities are usually asymptomatic and self-limited even with continuation of therapy. Complicating the attribution of liver test abnormalities to valacyclovir therapy is the fact that enzyme elevations are not uncommon during the course of varicella-zoster infection (both chickenpox and shingles) and can progress to clinically apparent hepatitis and even acute liver failure. Clinically apparent liver disease due to valacyclovir itself is rare, but isolated reports have been published. The time to onset was short (1 to 2 weeks) and the course mild, with few symptoms and rapid resolution (Case 1). The pattern of liver injury described was mixed hepatocellular-cholestatic. Immunoallergic features and autoantibodies were absent.
Likelihood score: D (possible rare cause of clinically apparent liver injury).
M Chen, V Vijay, Q Shi, Z Liu, H Fang, W Tong. FDA-Approved Drug Labeling for the Study of Drug-Induced Liver Injury, Drug Discovery Today, 16(15-16):697-703, 2011. PMID:21624500 DOI:10.1016/j.drudis.2011.05.007
M Chen, A Suzuki, S Thakkar, K Yu, C Hu, W Tong. DILIrank: the largest reference drug list ranked by the risk for developing drug-induced liver injury in humans. Drug Discov Today 2016, 21(4): 648-653. PMID:26948801 DOI:10.1016/j.drudis.2016.02.015
◉ Summary of Use during Lactation
The dosage of acyclovir in milk after valacyclovir is less than 1% of a typical infant dosage and would not be expected to cause any adverse effects in breastfed infants. No special precautions are required when using valacyclovir during breastfeeding. In one study, administration of valacyclovir to mothers with concurrent herpes simplex type 2 and HIV infections reduced breastmilk shedding of the HIV virus in breastmilk at 6 and 14 weeks postpartum, but not later. In another study in HIV-positive mothers, valacyclovir did not reduced breastmilk shedding of cytomegalovirus (CMV) or infant CMV acquisition.
◉ Effects in Breastfed Infants
In a study of pregnant women with concurrent HIV and Herpes simplex infections, mothers received zidovudine 300 mg daily from week of pregnancy until 12 months postpartum and nevirapine at delivery. Half of the women (n = 74) also received valacyclovir 500 mg orally twice daily from 34 weeks gestation until 12 months postpartum. At 6 weeks postpartum, all infants who received acyclovir in breastmilk had normal serum creatinine (<0.83 mg/dL). Their median serum creatinine and alanine aminotransferase (ALT) values, and growth were no different from those of unexposed infants, with the exception of one infant with an ALT level of 70.1 units/L. Infants whose mothers received valacyclovir generally had adverse effects that were similar to the placebo group, except that treated infants had a lower risk of eczema and oral thrush than infants in the placebo arm.
◉ Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=HDOVUKNUBWVHOX-QMMMGPOBSA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusValaciclovir [INN:BAN]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0124832264ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_useValaciclovirhttps://www.drugbank.ca/drugs/DB00577
- EPA DSSToxValacyclovirhttps://comptox.epa.gov/dashboard/DTXSID1023732CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeL-Valine, 2-((2-amino-1,6-dihydro-6-oxo-9H-purin-9- yl)methoxy)ethyl esterhttps://echa.europa.eu/substance-information/-/substanceinfo/100.114.479L-Valine, 2-((2-amino-1,6-dihydro-6-oxo-9H-purin-9- yl)methoxy)ethyl ester (EC: 603-015-6)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/40436Valaciclovir (EC: 658-047-3)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/189764
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Hazardous Substances Data Bank (HSDB)
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingValaciclovirhttp://www.hmdb.ca/metabolites/HMDB0014716HMDB0014716_msms_451694https://hmdb.ca/metabolites/HMDB0014716#spectra
- CCSbaseCCSbase Classificationhttps://ccsbase.net/
- ChEBI
- FDA Pharm ClassesLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingFDA Pharmacological Classificationhttps://www.fda.gov/ForIndustry/DataStandards/StructuredProductLabeling/ucm162549.htm
- LiverTox
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licenceVALACYCLOVIRhttps://platform.opentargets.org/drug/CHEMBL1349
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jsp
- Therapeutic Target Database (TTD)Valaciclovirhttps://idrblab.net/ttd/data/drug/details/D04QJD
- DailyMed
- Drug Induced Liver Injury Rank (DILIrank) DatasetLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Drugs and Lactation Database (LactMed)
- WHO Model Lists of Essential MedicinesLICENSEPermission from WHO is not required for the use of WHO materials issued under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Intergovernmental Organization (CC BY-NC-SA 3.0 IGO) license.https://www.who.int/about/policies/publishing/copyrightValaciclovirhttps://list.essentialmeds.org/medicines/563
- EU Clinical Trials Register
- National Drug Code (NDC) DirectoryLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/VALACICLOVIRNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlAnatomical Therapeutic Chemical (ATC) classificationhttp://www.genome.jp/kegg-bin/get_htext?br08303.kegAntiinfectiveshttp://www.genome.jp/kegg-bin/get_htext?br08307.keg
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- Metabolomics Workbench
- NIPH Clinical Trials Search of Japan
- WHO Anatomical Therapeutic Chemical (ATC) ClassificationLICENSEUse of all or parts of the material requires reference to the WHO Collaborating Centre for Drug Statistics Methodology. Copying and distribution for commercial purposes is not allowed. Changing or manipulating the material is not allowed.https://www.whocc.no/copyright_disclaimer/Valaciclovirhttps://www.whocc.no/atc_ddd_index/?code=J05AB11
- Protein Data Bank in Europe (PDBe)
- RCSB Protein Data Bank (RCSB PDB)LICENSEData files contained in the PDB archive (ftp://ftp.wwpdb.org) are free of all copyright restrictions and made fully and freely available for both non-commercial and commercial use. Users of the data should attribute the original authors of that structural data.https://www.rcsb.org/pages/policies
- Springer Nature
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- USGS Health-Based Screening Levels for Evaluating Water-Quality DataLICENSEhttps://www.usgs.gov/legal
- Wikidatavalacyclovirhttps://www.wikidata.org/wiki/Q418594
- WikipediaBoratabenzenehttps://en.wikipedia.org/wiki/BoratabenzeneValaciclovirhttps://en.wikipedia.org/wiki/Valaciclovir
- Wiley
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlValacyclovirhttps://www.ncbi.nlm.nih.gov/mesh/2028045Antiviral Agentshttps://www.ncbi.nlm.nih.gov/mesh/68000998
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403640139https://pubchem.ncbi.nlm.nih.gov/substance/403640139




