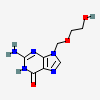
Acyclovir
- acyclovir
- Aciclovir
- 59277-89-3
- Acycloguanosine
- Zovirax
- Create:2019-01-10
- Modify:2025-01-18
Acyclovir is an antiviral prescription medicine approved by the U.S. Food and Drug Administration (FDA) to:
Treat and/or prevent the recurrence of certain types of herpes simplex virus (HSV) infections, including genital herpes
Treat varicella zoster virus (VZV) infections, including chicken pox (primary varicella infection) and shingles (herpes zoster)
Acyclovir is approved in different formulations and strengths for use in specific populations, including in people who are immunocompromised.
HSV and VZV infections can be opportunistic infections (OIs) of HIV.
 Valacyclovir (is active moiety of);
Valacyclovir (is active moiety of);  Valacyclovir Hydrochloride (active moiety of);
Valacyclovir Hydrochloride (active moiety of);  Acyclovir Sodium (active moiety of) ... View More ...
Acyclovir Sodium (active moiety of) ... View More ...
- 9-((2-Hydroxyethoxy)methyl)guanine
- Aci Sanorania
- Aci-Sanorania
- Acic
- Aciclobeta
- Aciclostad
- Aciclovir
- Aciclovir Alonga
- Aciclovir Sanorania
- aciclovir von ct
- Aciclovir-Sanorania
- Acifur
- Acipen Solutab
- Acivir
- Activir
- Acyclo V
- Acyclo-V
- Acycloguanosine
- Acyclovir
- Acyclovir Sodium
- Alonga, Aciclovir
- Antiherpes Creme
- Avirax
- Cicloferon
- Clonorax
- Cusiviral
- Genvir
- Herpetad
- Herpofug
- Herpotern
- Herpoviric
- Isavir
- Laciken
- Mapox
- Maynar
- Milavir
- Opthavir
- Sodium, Acyclovir
- Solutab, Acipen
- Supraviran
- Viclovir
- Vipral
- Virax Puren
- Virax-Puren
- ViraxPuren
- Virherpes
- Virmen
- Virolex
- Virupos
- Virzin
- Wellcome 248U
- Wellcome-248U
- Wellcome248U
- Zoliparin
- Zovirax
- Zyclir
- acyclovir
- Aciclovir
- 59277-89-3
- Acycloguanosine
- Zovirax
- Virorax
- Vipral
- Wellcome-248U
- 9-[(2-Hydroxyethoxy)methyl]guanine
- Aciclovirum
- Zovir
- Sitavig
- 2-Amino-9-((2-hydroxyethoxy)methyl)-1H-purin-6(9H)-one
- Gerpevir
- Hascovir
- Acyclovir-side chain-2-3H
- Aciclovirum [Latin]
- Acicloftal
- Cargosil
- Novirus
- 9-(2-Hydroxyethoxy)methylguanine
- 9-HYROXYETHOXYMETHYLGUANINE
- W-248-U
- 9-((2-Hydroxyethoxy)methyl)guanine
- Aciclovirum [INN-Latin]
- BW-248U
- DRG-0008
- MFCD00057880
- CCRIS 1953
- CHEBI:2453
- NSC 645011
- 141294-79-3
- HSDB 6511
- UNII-X4HES1O11F
- 2-Amino-1,9-dihydro-9-((2-hydroxyethoxy)methyl)-6H-purin-6-one
- EINECS 261-685-1
- X4HES1O11F
- Aciclovir [INN]
- NSC-645011
- NSC-758477
- AVACLYR
- 2-amino-9-(2-hydroxyethoxymethyl)-3H-purin-6-one
- DTXSID1022556
- Acyclovir (Aciclovir)
- 2-Amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-6H-purin-6-one
- 2-Amino-9-[(2-hydroxyethoxy)methyl]-1,9-dihydro-6H-purin-6-one
- 6H-Purin-6-one, 2-amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-
- ACYCLOVIR-SIDECHAIN-2-3H
- Acyclovir [USAN:USP]
- Zovirax (TN)
- NSC645011
- 9-[(2-Hydroxyethoxy)-methyl]guanine
- 2-amino-9-(2-hydroxyethoxymethyl)-1H-purin-6-one
- MLS000069633
- DTXCID102556
- Activir
- 9-(2-Hydroxyethoxymethyl)guanine
- 6H-Purin-6-one, 2-amino-1,9-dihydro-9-((2-hydroxyethoxy)methyl)-
- Aciclovirum (Latin)
- AC2
- Acyclovir [USAN]
- 2-amino-9-((2-hydroxyethoxy)methyl)-3H-purin-6(9H)-one
- NCGC00015061-02
- Aciclovier
- SMR000058225
- Genvir
- Maynar
- Zyclir
- Acyclovir (USAN:USP)
- ACICLOVIR (IARC)
- ACICLOVIR [IARC]
- 2-amino-9-[(2-hydroxyethoxy)methyl]-6,9-dihydro-3H-purin-6-one
- CAS-59277-89-3
- Aciclovirum (INN-Latin)
- 6H-Purin-6-one, 1,9-dihydro-2-amino-9-((2-hydroxyethoxy)methyl)-
- 2-amino-9-(2-hydroxyethoxymethyl)purin-6-ol
- ACICLOVIR (MART.)
- ACICLOVIR [MART.]
- ACYCLOVIR (USP-RS)
- ACYCLOVIR [USP-RS]
- ACICLOVIR (EP MONOGRAPH)
- ACICLOVIR [EP MONOGRAPH]
- ACYCLOVIR (USP MONOGRAPH)
- ACYCLOVIR [USP MONOGRAPH]
- Viropump
- AcycloFoam
- 2-amino-9-((2-hydroxyethoxy)methyl)-1,9-dihydro-6H-purin-6-one
- 2-amino-9-[(2-hydroxyethoxy)methyl]-6,9-dihydro-1H-purin-6-one
- Acic
- C8H11N5O3
- 2-amino-9-{[(2-hydroxyethyl)oxy]methyl}-1,9-dihydro-6H-purin-6-one
- Acyclo-V
- Acyclovir Lauriad
- Acyclovir (USP)
- 88713-38-6
- SR-01000075540
- ACV & Pluronic F-68
- B-LF& ACV
- VALACICLOVIR HYDROCHLORIDE IMPURITY B (EP IMPURITY)
- VALACICLOVIR HYDROCHLORIDE IMPURITY B [EP IMPURITY]
- Aclovir
- Acyclovir & Pluronic F-68
- Cyclovir
- Poviral
- Sitavir
- VALACICLOVIR HYDROCHLORIDE HYDRATE IMPURITY B (EP IMPURITY)
- VALACICLOVIR HYDROCHLORIDE HYDRATE IMPURITY B [EP IMPURITY]
- Prestwick_6
- 1pwy
- BW-248-U
- Sitavig (TN)
- Acyclovir (Standard)
- Spectrum_001739
- ACICLOVIR [JAN]
- ACYCLOVIR [MI]
- ACYCLOVIR [HSDB]
- Opera_ID_1674
- Prestwick0_000086
- Prestwick1_000086
- Prestwick2_000086
- Prestwick3_000086
- Spectrum2_001563
- Spectrum3_001874
- Spectrum4_000225
- Spectrum5_001093
- ACYCLOVIR [VANDF]
- Lopac-A-4669
- Aciclovir (JP18/INN)
- CHEMBL184
- A 4669
- ACICLOVIR [WHO-DD]
- ACICLOVIR [WHO-IP]
- SCHEMBL3175
- Lopac0_000037
- ACICLOVIRUM [WHO-IP]
- BSPBio_000012
- BSPBio_003348
- KBioGR_000889
- KBioSS_002219
- BIDD:GT0646
- DivK1c_000185
- SPECTRUM1503603
- SPBio_001466
- SPBio_001951
- 2-amino-9-[(2-hydroxyethoxy)methyl]hydropurin-6-one
- BPBio1_000014
- GTPL4829
- SCHEMBL9828560
- 9(2-hydroxyethoxymethyl)guanine
- ACYCLOVIR [ORANGE BOOK]
- HMS500J07
- KBio1_000185
- KBio2_002219
- KBio2_004787
- KBio2_007355
- KBio3_002850
- D06BB03
- J05AB01
- S01AD03
- NINDS_000185
- GLXC-04946
- HMS1568A14
- HMS1922E08
- HMS2090G09
- HMS2095A14
- HMS2234K21
- HMS3259N10
- HMS3260G15
- HMS3269M15
- HMS3372K02
- HMS3413D21
- HMS3655C14
- HMS3677D21
- HMS3712A14
- Pharmakon1600-01503603
- 2-amino-9-[(2-hydroxyethoxy)methyl]-3,9-dihydro-6H-purin-6-one
- BCP11036
- 9-(2-hydroxyethoxy methyl) guanine
- Tox21_110075
- Tox21_500037
- 69657-51-8 (Na salt)
- BBL009642
- BDBM50021776
- BDBM50103518
- CCG-39909
- HY-17422R
- NSC335752
- NSC758477
- NSC780378
- s1807
- STK796771
- STL257059
- STL301862
- AKOS000656213
- AKOS015995680
- AKOS022135433
- Tox21_110075_1
- AC-8068
- CS-1353
- DB00787
- KS-1027
- LP00037
- NC00717
- NSC-780378
- SDCCGSBI-0050026.P003
- Bovine lactoferrin & 2-Amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-6H-purin-6-one
- IDI1_000185
- SMP1_000007
- NCGC00015061-01
- NCGC00015061-03
- NCGC00015061-04
- NCGC00015061-05
- NCGC00015061-06
- NCGC00015061-07
- NCGC00015061-08
- NCGC00015061-09
- NCGC00015061-10
- NCGC00015061-12
- NCGC00015061-13
- NCGC00015061-28
- NCGC00015061-29
- NCGC00022426-03
- NCGC00093555-01
- NCGC00093555-02
- NCGC00093555-03
- NCGC00093555-04
- NCGC00167756-01
- NCGC00167756-02
- NCGC00260722-01
- NCGC00381719-03
- HY-17422
- SY051130
- Acycloguanosine, >=99% (HPLC), powder
- Aciclovir 1.0 mg/ml in Dimethyl Sulfoxide
- A1915
- EU-0100037
- NS00009000
- SW196324-3
- C06810
- D00222
- EN300-123010
- A832236
- Q147101
- Q-200591
- SR-01000075540-1
- SR-01000075540-3
- SR-01000075540-5
- 2-amino-9-[(2-hydroxyethoxy)methyl]-9H-purin-6-ol
- 2-azanyl-9-(2-hydroxyethyloxymethyl)-3H-purin-6-one
- BRD-K32318651-001-17-9
- BRD-K32318651-001-21-1
- BRD-K32318651-001-22-9
- BRD-K32318651-001-23-7
- BRD-K32318651-001-24-5
- Aciclovir, British Pharmacopoeia (BP) Reference Standard
- F2173-0946
- Z1546610471
- Aciclovir, European Pharmacopoeia (EP) Reference Standard
- 2-Amino-9-(2-hydroxy-ethoxymethyl)-5,9-dihydro-purin-6-one
- Acyclovir, United States Pharmacopeia (USP) Reference Standard
- 2-Amino-9-(2-hydroxy-ethoxymethyl)-5,9-dihydro-purin-6-one (ACV)
- 2-Amino-9-[(2-hydroxyethoxy)methyl]-1,9-dihydro-6H-purin-6-one #
- ACV; Acycloguanosine; Acyclovir; NSC 645011; NSC-645011; NSC645011
- Acyclovir, Pharmaceutical Secondary Standard; Certified Reference Material
- Aciclovir for peak identification 1, European Pharmacopoeia (EP) Reference Standard
- Aciclovir for peak identification 2, European Pharmacopoeia (EP) Reference Standard
- Aciclovir for system suitability, European Pharmacopoeia (EP) Reference Standard
- InChI=1/C8H11N5O3/c9-8-11-6-5(7(15)12-8)10-3-13(6)4-16-2-1-14/h3,14H,1-2,4H2,(H3,9,11,12,15
155.82 Ų [M+Na]+ [CCS Type: TW; Method: calibrated with polyalanine and drug standards]
145.91 Ų [M+H]+ [CCS Type: TW; Method: calibrated with polyalanine and drug standards]
155.98 Ų [M+K]+ [CCS Type: TW; Method: calibrated with polyalanine and drug standards]
Antiherpes medicines
Ophthalmological preparations > Anti-infective agents
232.168594 65432
230.152679 53624
380.257111 44764
133.063354 42168
148.074387 33700
224.2 100
141.8 0.52
141.4 0.18
150.2 0.06
162.3 0.06
226.0935 999
227.0974 104
226.0971 999
165.0528 914
Valacyclovir (is active moiety of)
Valacyclovir Hydrochloride (active moiety of)
Acyclovir Sodium (active moiety of)
- Acyclovir; hydrocortisone (component of)
Acyclovir is an antiviral prescription medicine approved by the U.S. Food and Drug Administration (FDA) to:
Treat and/or prevent the recurrence of certain types of herpes simplex virus (HSV) infections, including genital herpes
Treat varicella zoster virus (VZV) infections, including chicken pox (primary varicella infection) and shingles (herpes zoster)
Acyclovir is approved in different formulations and strengths for use in specific populations, including in people who are immunocompromised.
HSV and VZV infections can be opportunistic infections (OIs) of HIV.
D - Dermatologicals
D06 - Antibiotics and chemotherapeutics for dermatological use
D06B - Chemotherapeutics for topical use
D06BB - Antivirals
D06BB03 - Aciclovir
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AB - Nucleosides and nucleotides excl. reverse transcriptase inhibitors
J05AB01 - Aciclovir
- Cytoplasm
- Membrane
Use (kg) in Switzerland (2009): >250
Use (kg; approx.) in Germany (2009): >10000
Use (kg; exact) in Germany (2009): 15729
Use (kg) in USA (2002): 21300
Consumption (g per capita) in Switzerland (2009): 0.032
Consumption (g per capita; approx.) in Germany (2009): 0.12
Consumption (g per capita; exact) in Germany (2009): 0.19
Consumption (g per capita) in the USA (2002): 0.076
Excretion rate: 0.9
Calculated removal (%): 22

H315 (77.9%): Causes skin irritation [Warning Skin corrosion/irritation]
H319 (77.9%): Causes serious eye irritation [Warning Serious eye damage/eye irritation]
H335 (71.6%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation]
P261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 95 reports by companies from 20 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Reported as not meeting GHS hazard criteria per 14 of 95 reports by companies. For more detailed information, please visit ECHA C&L website.
There are 18 notifications provided by 81 of 95 reports by companies with hazard statement code(s).
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Skin Irrit. 2 (77.9%)
Eye Irrit. 2A (77.9%)
STOT SE 3 (71.6%)
Acute Tox. 4 (100%)
Acute Tox. 4 (100%)
Carc. 2 (100%)
IMAP assessments - 6H-Purin-6-one, 2-amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-: Environment tier I assessment
IMAP assessments - 6H-Purin-6-one, 2-amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-: Human health tier I assessment
Despite widespread use, there is little evidence that acyclovir when given orally causes significant liver injury. Serum enzyme levels generally do not change during oral acyclovir therapy. High dose intravenous administration of acyclovir is associated with renal dysfunction and thrombocytopenia, and occasionally with transient mild-to-moderate elevations in serum ALT levels, which have been asymptomatic and self-limited. There have rare instances of acute, clinically apparent liver injury reported that were attributed to acyclovir or valacyclovir (a prodrug of acyclovir with better oral absorption), but these have not been particularly convincing. Some degree of liver injury and even jaundice can occur during the course of herpes simplex or varicella zoster infection, and these complications could be mistaken for drug induced liver injury. Furthermore, in the reported cases, patients were receiving other medications and had other unlying comorbidities that may have been responsible for the liver injury.
Likelihood score: D (possible rare cause of clinically apparent liver injury).
M Chen, V Vijay, Q Shi, Z Liu, H Fang, W Tong. FDA-Approved Drug Labeling for the Study of Drug-Induced Liver Injury, Drug Discovery Today, 16(15-16):697-703, 2011. PMID:21624500 DOI:10.1016/j.drudis.2011.05.007
M Chen, A Suzuki, S Thakkar, K Yu, C Hu, W Tong. DILIrank: the largest reference drug list ranked by the risk for developing drug-induced liver injury in humans. Drug Discov Today 2016, 21(4): 648-653. PMID:26948801 DOI:10.1016/j.drudis.2016.02.015
◉ Summary of Use during Lactation
Even with the highest maternal dosages, the dosage of acyclovir in milk is only about 1% of a typical infant dosage and would not be expected to cause any adverse effects in breastfed infants. Topical acyclovir applied to small areas of the mother's body away from the breast should pose no risk to the infant. Only water-miscible cream or gel products should be applied to the breast because ointments may expose the infant to high levels of mineral paraffins via licking.
◉ Effects in Breastfed Infants
The mother of a 4-month-old infant noticed no adverse effects in her breastfed infant while she was taking an acyclovir dosage of 800 mg orally 5 times daily.
◉ Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=MKUXAQIIEYXACX-UHFFFAOYSA-N
- Drink plenty of fluids. Dehydration with acyclovir predisposes patients to nephrotoxicity.
- Take with or without food. The absorption is unaffected by food.
- Australian Industrial Chemicals Introduction Scheme (AICIS)6H-Purin-6-one, 2-amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-https://services.industrialchemicals.gov.au/search-assessments/
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusAcyclovir [USAN:USP]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0059277893ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_use
- DTP/NCILICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuse
- EPA DSSToxCompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-notice2-amino-9-[(2-hydroxyethoxy)methyl]-6,9-dihydro-1H-purin-6-onehttps://echa.europa.eu/substance-information/-/substanceinfo/100.159.615Aciclovir (EC: 261-685-1)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/263652-amino-9-[(2-hydroxyethoxy)methyl]-6,9-dihydro-1H-purin-6-one (EC: 631-369-1)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/164434
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Hazardous Substances Data Bank (HSDB)
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingHMDB0014925_msms_374776https://hmdb.ca/metabolites/HMDB0014925#spectra
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/
- CCSbaseCCSbase Classificationhttps://ccsbase.net/
- ChEBI
- Drug Database, Clinicalinfo.hiv.govLICENSEUnless otherwise noted, material presented on the HIV.gov website is considered Federal government information and is in the public domain. That means this information may be freely copied and distributed. We request that you use appropriate attribution to HIV.gov.https://www.hiv.gov/about-us/mission-and-team
- FDA Pharm ClassesLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingFDA Pharmacological Classificationhttps://www.fda.gov/ForIndustry/DataStandards/StructuredProductLabeling/ucm162549.htm
- LiverTox
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/LOTUS Treehttps://lotus.naturalproducts.net/
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licence
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jsp
- Drug Gene Interaction database (DGIdb)LICENSEThe data used in DGIdb is all open access and where possible made available as raw data dumps in the downloads section.http://www.dgidb.org/downloads
- Therapeutic Target Database (TTD)
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/ACYCLOVIRNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- Crystallography Open Database (COD)LICENSEAll data in the COD and the database itself are dedicated to the public domain and licensed under the CC0 License. Users of the data should acknowledge the original authors of the structural data.https://creativecommons.org/publicdomain/zero/1.0/
- The Cambridge Structural Database
- DailyMed
- Drug Induced Liver Injury Rank (DILIrank) DatasetLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- European Medicines Agency (EMA)LICENSEInformation on the European Medicines Agency's (EMA) website is subject to a disclaimer and copyright and limited reproduction notices.https://www.ema.europa.eu/en/about-us/legal-notice
- Drugs and Lactation Database (LactMed)
- Drugs@FDALICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- WHO Model Lists of Essential MedicinesLICENSEPermission from WHO is not required for the use of WHO materials issued under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Intergovernmental Organization (CC BY-NC-SA 3.0 IGO) license.https://www.who.int/about/policies/publishing/copyright
- EU Clinical Trials Register
- FDA Orange BookLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- National Drug Code (NDC) DirectoryLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawAcyclovirhttp://www.nist.gov/srd/nist1a.cfm
- International Agency for Research on Cancer (IARC)LICENSEMaterials made available by IARC/WHO enjoy copyright protection under the Berne Convention for the Protection of Literature and Artistic Works, under other international conventions, and under national laws on copyright and neighbouring rights. IARC exercises copyright over its Materials to make sure that they are used in accordance with the Agency's principles. All rights are reserved.https://publications.iarc.fr/Terms-Of-UseIARC Classificationhttps://www.iarc.fr/
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlTherapeutic category of drugs in Japanhttp://www.genome.jp/kegg-bin/get_htext?br08301.kegUSP drug classificationhttp://www.genome.jp/kegg-bin/get_htext?br08302.kegAnatomical Therapeutic Chemical (ATC) classificationhttp://www.genome.jp/kegg-bin/get_htext?br08303.kegAntiinfectiveshttp://www.genome.jp/kegg-bin/get_htext?br08307.kegDrugs listed in the Japanese Pharmacopoeiahttp://www.genome.jp/kegg-bin/get_htext?br08311.kegRisk category of Japanese OTC drugshttp://www.genome.jp/kegg-bin/get_htext?br08312.kegClassification of Japanese OTC drugshttp://www.genome.jp/kegg-bin/get_htext?br08313.keg
- MassBank Europe
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- Metabolomics Workbench
- NIPH Clinical Trials Search of Japan
- SpectraBase2-amino-9-((2-hydroxyethoxy)methyl)-1H-purin-6(9H)-onehttps://spectrabase.com/spectrum/4IEXY20hQyPAcycloguanosinehttps://spectrabase.com/spectrum/9BwhQHUAOkUAcycloguanosinehttps://spectrabase.com/spectrum/3vqVOVCbWEw9-[(2-HYDROXYETHOXY)-METHYL]-GUANINE;ACYCLOVIRhttps://spectrabase.com/spectrum/9i5uMToolCZMKUXAQIIEYXACX-UHFFFAOYSA-Nhttps://spectrabase.com/spectrum/CfmK5x2hMQr
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.html
- NMRShiftDB
- WHO Anatomical Therapeutic Chemical (ATC) ClassificationLICENSEUse of all or parts of the material requires reference to the WHO Collaborating Centre for Drug Statistics Methodology. Copying and distribution for commercial purposes is not allowed. Changing or manipulating the material is not allowed.https://www.whocc.no/copyright_disclaimer/
- Protein Data Bank in Europe (PDBe)
- RCSB Protein Data Bank (RCSB PDB)LICENSEData files contained in the PDB archive (ftp://ftp.wwpdb.org) are free of all copyright restrictions and made fully and freely available for both non-commercial and commercial use. Users of the data should attribute the original authors of that structural data.https://www.rcsb.org/pages/policies
- Springer Nature
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- USGS Health-Based Screening Levels for Evaluating Water-Quality DataLICENSEhttps://www.usgs.gov/legal
- Wikidata6H-Purin-6-one, 2-amino-1,9-dihydro-9-[(2-hydroxyethoxy-2-t)methyl]-https://www.wikidata.org/wiki/Q90542928
- Wikipedia
- Wiley
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlAntiviral Agentshttps://www.ncbi.nlm.nih.gov/mesh/68000998
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403411460https://pubchem.ncbi.nlm.nih.gov/substance/403411460