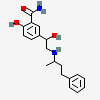
Labetalol
- labetalol
- 36894-69-6
- Labetolol
- Ibidomide
- Albetol
- Create:2005-03-25
- Modify:2025-01-18
 Labetalol Hydrochloride (has salt form).
Labetalol Hydrochloride (has salt form).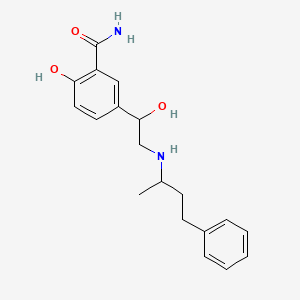
- AH 5158
- AH-5158
- AH5158
- Albetol
- Apo Labetalol
- Apo-Labetalol
- ApoLabetalol
- Dilevalol
- Hydrochloride, Labetalol
- Labetalol
- Labetalol Hydrochloride
- Labetalol, (R,R)-Isomer
- Labetolol
- Normodyne
- Presolol
- R,R Labetalol
- R,R-Labetalol
- SCH 19927
- SCH-19927
- SCH19927
- Trandate
- labetalol
- 36894-69-6
- Labetolol
- Ibidomide
- Albetol
- Labetalolum
- Normodyne
- 2-Hydroxy-5-(1-hydroxy-2-((4-phenylbutan-2-yl)amino)ethyl)benzamide
- Labetalolum [INN-Latin]
- AH 5158
- 5-(1-Hydroxy-2-(1-methyl-3-phenylpropylamino)ethyl)salicylamide
- 2-hydroxy-5-[1-hydroxy-2-(4-phenylbutan-2-ylamino)ethyl]benzamide
- HSDB 6537
- EINECS 253-258-3
- Labetalol (INN)
- 3-Carboxamido-4-hydroxy-alpha-((1-methyl-3-phenylpropylamino)methyl)benzyl alcohol
- AH-5158A FREE BASE
- CHEBI:6343
- UNII-R5H8897N95
- SCH-15719W FREE BASE
- 2-Hydroxy-5-(1-hydroxy-2-((1-methyl-3-phenylpropyl)amino)ethyl)benzamide
- Benzamide, 2-hydroxy-5-(1-hydroxy-2-((1-methyl-3-phenylpropyl)amino)ethyl)-
- R5H8897N95
- 2-hydroxy-5-{1-hydroxy-2-[(4-phenylbutan-2-yl)amino]ethyl}benzamide
- DTXSID2023191
- EC 253-258-3
- 36894-69-6 (free base)
- Benzamide, 2-hydroxy-5-[1-hydroxy-2-[(1-methyl-3-phenylpropyl)amino]ethyl]-
- LABETALOL [INN]
- Labetalolum (INN-Latin)
- Labetalol [INN:BAN]
- 2-hydroxy-5-{1-hydroxy-2-[(1-methyl-3-phenylpropyl)amino]ethyl}benzamide
- kabetalol
- labetalolo
- 2-Hydroxy-5-(1-hydroxy-2-[(1-methyl-3-phenylpropyl)amino]ethyl)benzamide
- MFCD00242941
- Labetalol (Standard)
- Amipress (Salt/Mix)
- Trandate (Salt/Mix)
- Normodyne (Salt/Mix)
- Spectrum_001607
- LABETALOL [MI]
- LABETALOL [HSDB]
- Prestwick0_000277
- Prestwick1_000277
- Prestwick2_000277
- Prestwick3_000277
- Spectrum2_000863
- Spectrum3_001581
- Spectrum4_000184
- Spectrum5_001010
- LABETALOL [VANDF]
- CHEMBL429
- LABETALOL [WHO-DD]
- SCHEMBL4582
- Lopac0_000687
- BSPBio_000154
- BSPBio_003142
- KBioGR_000727
- KBioSS_002087
- DivK1c_000474
- SPBio_000905
- SPBio_002373
- BPBio1_000170
- DTXCID603191
- GTPL7207
- BDBM25758
- KBio1_000474
- KBio2_002087
- KBio2_004655
- KBio2_007223
- KBio3_002642
- C07AG01
- CHEBI:167638
- NINDS_000474
- Labetalol hydrochloride (Salt/Mix)
- BCP31095
- AH5158;Sch-15719W free base
- AKOS015908406
- CCG-204772
- DB00598
- DS-4652
- HY-121383R
- SDCCGSBI-0050665.P004
- IDI1_000474
- NCGC00015595-03
- NCGC00015595-04
- NCGC00015595-06
- NCGC00015595-07
- NCGC00015595-12
- NCGC00015595-14
- NCGC00015595-19
- NCGC00089800-02
- AC-18747
- Labetolol;Albetol;Normodyne;Apo-Labetalol
- SBI-0050665.P003
- HY-121383
- AB00053659
- CS-0081835
- NS00008578
- C07063
- C74903
- D08106
- AB00053659_13
- EN300-1272609
- A823444
- L001344
- Q958087
- Q-201273
- BRD-A07440155-003-05-6
- BRD-A07440155-003-16-3
- BRD-A07440155-003-25-4
- 5-(1-Hydroxy-2-((1-methyl-3-phenylpropyl)amino)ethyl)salicylamide
- 2-hydroxy-5-{1-hydroxy-2-[(4-phenylbutan-2-yl)amino]ethyl}benzene-1-carboximidic acid
- 3-Carboxamido-4-hydroxy-.alpha.-((1-methyl-3-phenylpropylamino)methyl)benzyl alcohol
- 32780-40-8
175.22 Ų [M+Na]+ [CCS Type: TW; Method: calibrated with polyalanine and drug standards]
173.29 Ų [M+H-H2O]+ [CCS Type: TW; Method: calibrated with polyalanine and drug standards]
177.42 Ų [M+K]+ [CCS Type: TW; Method: calibrated with polyalanine and drug standards]
176.41 Ų [M+H]+ [CCS Type: TW; Method: calibrated with polyalanine and drug standards]
157.0408 100
175.0513 37.22
131.0377 32.65
181.0381 13.70
163.0275 12.59
175.0513 100
157.0407 86.19
176.0591 59.27
131.0376 51.58
181.0381 36.78
329.1858 999
311.1756 537
330.1892 223
312.1783 107
331.1917 28
311.1748 999
162.0536 326
294.148 279
207.1116 214
179.0802 190
 Labetalol Hydrochloride (has salt form)
Labetalol Hydrochloride (has salt form)Use (kg) in USA (2002): 1980
Consumption (g per capita) in the USA (2002): 0.00702
Excretion rate: 0.04
Calculated removal (%): 32.1



H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation]
H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation]
H335 (100%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation]
H361 (100%): Suspected of damaging fertility or the unborn child [Warning Reproductive toxicity]
H411 (66.7%): Toxic to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard]
P203, P261, P264, P264+P265, P271, P273, P280, P302+P352, P304+P340, P305+P351+P338, P318, P319, P321, P332+P317, P337+P317, P362+P364, P391, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 3 reports by companies from 2 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Skin Irrit. 2 (100%)
Eye Irrit. 2 (100%)
STOT SE 3 (100%)
Repr. 2 (100%)
Aquatic Chronic 2 (66.7%)
Labetalol therapy has been associated with mild-to-moderate elevations of serum aminotransferase levels in up to 8% of patients, a rate far higher than with other beta-blockers. These elevations, however, are usually transient, not associated with symptoms, and can resolve even with continuation of therapy. Idiosyncratic, clinically apparent liver injury from labetalol is rare, but several instances have been reported as isolated case reports as well as in case series. The liver injury typically arises after 4 to 16 weeks of therapy and the pattern of serum enzyme elevations is usually hepatocellular with an acute hepatitis-like onset and course. Immunoallergic features (rash, fever, eosinophilia) are uncommon as is autoantibody formation. While most cases resolve rapidly once labetalol is stopped, there have been several instances of acute liver failure and death or need for emergency liver transplantation associated with labetalol use, particularly if there is a delay in its discontinuation. Labetalol is the beta-blocker with the highest apparent risk for causing clinically apparent liver injury.
Likelihood score: C (probable although rare cause of clinically apparent liver injury).
M Chen, V Vijay, Q Shi, Z Liu, H Fang, W Tong. FDA-Approved Drug Labeling for the Study of Drug-Induced Liver Injury, Drug Discovery Today, 16(15-16):697-703, 2011. PMID:21624500 DOI:10.1016/j.drudis.2011.05.007
M Chen, A Suzuki, S Thakkar, K Yu, C Hu, W Tong. DILIrank: the largest reference drug list ranked by the risk for developing drug-induced liver injury in humans. Drug Discov Today 2016, 21(4): 648-653. PMID:26948801 DOI:10.1016/j.drudis.2016.02.015
◉ Summary of Use during Lactation
Because of the low levels of labetalol in breastmilk, amounts ingested by the infant are small and would not be expected to cause any adverse effects in fullterm breastfed infants. No special precautions are required in most infants. However, other agents may be preferred while nursing a preterm infant. Labetalol may predispose nursing mothers to Raynaud’s phenomenon of the nipple.
◉ Effects in Breastfed Infants
One investigator reported that no adverse effects occurred in breastfed infants whose mothers were taking labetalol in doses of 330 to 800 mg daily.
A 26-week premature infant weighing 640 grams developed sinus bradycardia (80 to 90 bpm) and isolated atrial premature beats after nasogastric feeding with mother's pumped breastmilk began on day 8 of life. The mother was taking labetalol 300 mg twice daily by mouth for hypertension. Bradycardia and premature beats resolved within 24 hours of substitution of formula for breastmilk. No other causes for bradycardia could be identified. One untimed sample of the mother's breastmilk contained 710 mcg/L of labetalol. Although the authors estimated the infant's dose to be 100 mg/kg daily, a recalculation using their data indicates that the infant's dose was only 100 mcg/kg daily.
A 2-month-old infant was being breastfed exclusively by a mother taking labetalol 100 mg twice daily. The infants electrocardiogram had a regular heart rate, but borderline prolonged QT. The infant was started on propranolol 1 mg/kg daily for infantile hemangioma. One month later, the infant had a normal QT interval. A second infant was exclusively breastfed by a mother taking labetalol 150 mg twice daily and nifedipine 60 mg daily. The infant was started on propranolol 0.6 mg/kg daily for infantile hemangioma. The propranolol dose was increased over 2 weeks to 3.4 mg/kg daily. The infant had some sleeping difficulties with the higher propranolol dose, but no other symptoms.
A prospective study of pregnant patients taking a beta-blocker asked mothers to complete a questionnaire about postpartum breastfeeding and any side effects in their breastfed infants. One mother reported taking labetalol in an unreported dosage while breastfeeding. She reported weak sucking in her infant.
◉ Effects on Lactation and Breastmilk
Intravenous labetalol can increase serum prolactin in men and non-nursing women, although the increase is greater in women. Oral labetalol does not increase serum prolactin. The maternal prolactin level in a mother with established lactation may not affect her ability to breastfeed.
A woman with a history of symptoms of Raynaud's phenomenon developed Raynaud's phenomenon of the nipples when treated for pregnancy-induced hypertension with labetalol 100 mg twice daily. She breastfed for 5 weeks, but nursing caused pain in her nipples. In a subsequent pregnancy, similar symptoms occurred during treatment with labetalol 100 mg twice daily. Discontinuing labetalol eliminated the nipple pain in both instances.
A pregnant woman was treated on two occasions with intravenous labetalol for pre-eclampsia. On each occasion, she reported a burning sensation of the nipples. While continuing on labetalol, sustained-release nifedipine was added to her regimen and the burning of the nipple did not return.
◈ What is labetalol?
Labetalol is a medication that has been used to treat high blood pressure and chest pain. It works by slowing the heart rate and opening blood vessels to improve blood flow and lower blood pressure. Labetalol is part of a group of medications called beta-blockers. Some brand names are Trandate®, Normodyne®, or Labrocol®.Sometimes when people find out they are pregnant, they think about changing how they take their medication, or stopping their medication altogether. However, it is important to talk with your healthcare providers before making any changes to how you take this medication. Your healthcare providers can talk with you about the benefits of treating your condition and the risks of untreated illness during pregnancy.
◈ I take labetalol. Can it make it harder for me to get pregnant?
It is not known if labetalol can make it harder to get pregnant.
◈ Does taking labetalol increase the chance for miscarriage?
Miscarriage is common and can occur in any pregnancy for many different reasons. Based on the studies reviewed, it is not known if labetalol can increase the chance for miscarriage.
◈ Does taking labetalol increase the chance of birth defects?
Every pregnancy starts out with a 3-5% chance of having a birth defect. This is called the background risk. Information on the use of labetalol in pregnancy is limited. Available information does not suggest the use of labetalol in pregnancy increases the chance of birth defects.
◈ Does taking labetalol in pregnancy increase the chance of other pregnancy-related problems?
Most studies do not suggest that labetalol increases the chance of other pregnancy-related problems, such as preterm delivery (birth before week 37), low birth weight (weighing less than 5 pounds, 8 ounces [2500 grams] at birth), or stillbirth.There have been a few reports of temporary symptoms of beta-blockade that appeared shortly after birth in infants who were exposed to labetalol in late pregnancy. Symptoms can include slowed heart rate, low blood pressure, and low blood sugar. If these symptoms occur, they are expected to pass within 3 days.There have been cases where the effects of beta-blocker exposure occurred a week after birth. The symptoms were more severe and life-threatening. Symptoms reported included abnormal breathing, sepsis (blood infection), and seizures. It has been suggested that preterm infants that were exposed to labetalol over for a long period of time during pregnancy should be carefully monitored during the first week after birth.
◈ Does taking labetalol in pregnancy affect future behavior or learning for the child?
Based on the studies reviewed, it is not known if labetalol increases the chance for behavior or learning issues. One study of 32 children between the ages of 3-7 years old who were exposed to labetalol during pregnancy found no differences on formal testing of learning and behavior compared to children who were not exposed to labetalol. Another study found a higher chance for attention deficit hyperactivity disorder (ADHD) in children who were exposed to labetalol or a different type of high blood pressure medication during pregnancy.Breastfeeding while taking labetalol:Labetalol gets into breastmilk in small amounts and is not expected to cause problems in full-term breastfed infants. Be sure to talk to your healthcare provider about all of your breastfeeding questions.
◈ If a male takes labetalol, could it affect fertility (ability to get partner pregnant) or increase the chance of birth defects?
There have been case reports of sexual dysfunction (trouble with ejaculation) in males while taking labetalol. This can make it harder to conceive a pregnancy. Studies have not been done to see if labetalol could increase the chance of birth defects above the background risk. In general, exposures that fathers or sperm donors have are unlikely to increase risks to a pregnancy. For more information, please see the MotherToBaby fact sheet Paternal Exposures at https://mothertobaby.org/fact-sheets/paternal-exposures-pregnancy/.
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=SGUAFYQXFOLMHL-UHFFFAOYSA-N
- Australian Industrial Chemicals Introduction Scheme (AICIS)Benzamide, 2-hydroxy-5-[1-hydroxy-2-[(1-methyl-3-phenylpropyl)amino]ethyl]-https://services.industrialchemicals.gov.au/search-assessments/
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusLabetalol [INN:BAN]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0036894696ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_use
- EPA DSSToxCompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeLabetalol (EC: 253-258-3)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/20871
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Hazardous Substances Data Bank (HSDB)
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingHMDB0014736_msms_2226277https://hmdb.ca/metabolites/HMDB0014736#spectra
- BindingDBLICENSEAll data curated by BindingDB staff are provided under the Creative Commons Attribution 3.0 License (https://creativecommons.org/licenses/by/3.0/us/).https://www.bindingdb.org/rwd/bind/info.jsp2-hydroxy-5-{1-hydroxy-2-[(4-phenylbutan-2-yl)amino]ethyl}benzamidehttps://www.bindingdb.org/rwd/bind/chemsearch/marvin/MolStructure.jsp?monomerid=25758
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jsp
- Drug Gene Interaction database (DGIdb)LICENSEThe data used in DGIdb is all open access and where possible made available as raw data dumps in the downloads section.http://www.dgidb.org/downloads
- IUPHAR/BPS Guide to PHARMACOLOGYLICENSEThe Guide to PHARMACOLOGY database is licensed under the Open Data Commons Open Database License (ODbL) https://opendatacommons.org/licenses/odbl/. Its contents are licensed under a Creative Commons Attribution-ShareAlike 4.0 International License (http://creativecommons.org/licenses/by-sa/4.0/)https://www.guidetopharmacology.org/about.jsp#licenseGuide to Pharmacology Target Classificationhttps://www.guidetopharmacology.org/targets.jsp
- Toxin and Toxin Target Database (T3DB)LICENSET3DB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (T3DB) and the original publication.http://www.t3db.ca/downloadsLabetalolhttp://www.t3db.ca/toxins/T3D3517
- CCSbaseCCSbase Classificationhttps://ccsbase.net/
- ChEBI
- FDA Pharm ClassesLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingFDA Pharmacological Classificationhttps://www.fda.gov/ForIndustry/DataStandards/StructuredProductLabeling/ucm162549.htm
- LiverTox
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licence
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- Therapeutic Target Database (TTD)
- DailyMed
- Drug Induced Liver Injury Rank (DILIrank) DatasetLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Drugs and Lactation Database (LactMed)
- Mother To Baby Fact SheetsLICENSECopyright by OTIS. This work is available under the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported license (CC BY-NC-ND 3.0).https://www.ncbi.nlm.nih.gov/books/about/copyright/
- Drugs@FDALICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/LABETALOLNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- EU Clinical Trials Register
- National Drug Code (NDC) DirectoryLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawLabetalolhttp://www.nist.gov/srd/nist1a.cfm
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlAnatomical Therapeutic Chemical (ATC) classificationhttp://www.genome.jp/kegg-bin/get_htext?br08303.kegTarget-based classification of drugshttp://www.genome.jp/kegg-bin/get_htext?br08310.keg
- MassBank Europe
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- Metabolomics Workbench
- SpectraBase2-Hydroxy-5-(1-hydroxy-2-[(1-methyl-3-phenylpropyl)amino]ethyl)benzamidehttps://spectrabase.com/spectrum/7Jz6JBjkdVf
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.html
- WHO Anatomical Therapeutic Chemical (ATC) ClassificationLICENSEUse of all or parts of the material requires reference to the WHO Collaborating Centre for Drug Statistics Methodology. Copying and distribution for commercial purposes is not allowed. Changing or manipulating the material is not allowed.https://www.whocc.no/copyright_disclaimer/
- PharmGKBLICENSEPharmGKB data are subject to the Creative Commons Attribution-ShareALike 4.0 license (https://creativecommons.org/licenses/by-sa/4.0/).https://www.pharmgkb.org/page/policies
- PharosLICENSEData accessed from Pharos and TCRD is publicly available from the primary sources listed above. Please respect their individual licenses regarding proper use and redistribution.https://pharos.nih.gov/about
- Springer Nature
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidata
- WikipediaNesiritidehttps://en.wikipedia.org/wiki/Nesiritide
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlAntihypertensive Agentshttps://www.ncbi.nlm.nih.gov/mesh/68000959Adrenergic alpha-1 Receptor Antagonistshttps://www.ncbi.nlm.nih.gov/mesh/68058668Adrenergic beta-Antagonistshttps://www.ncbi.nlm.nih.gov/mesh/68000319Sympathomimeticshttps://www.ncbi.nlm.nih.gov/mesh/68013566
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403462314https://pubchem.ncbi.nlm.nih.gov/substance/403462314
- NCBI