Quinidine
- quinidine
- 56-54-2
- (+)-Quinidine
- Conquinine
- Pitayine
- Create:2005-06-24
- Modify:2025-01-11
 Quinidine Gluconate (has salt form);
Quinidine Gluconate (has salt form);  Quinidine Sulfate (active moiety of);
Quinidine Sulfate (active moiety of);  Quinidine bisulfate (is active moiety of) ... View More ...
Quinidine bisulfate (is active moiety of) ... View More ...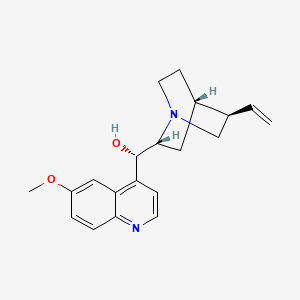
- Adaquin
- Apo Quinidine
- Apo-Quinidine
- Chinidin
- Quincardine
- Quinidex
- Quinidine
- Quinidine Sulfate
- Quinora
- Sulfate, Quinidine
- quinidine
- 56-54-2
- (+)-Quinidine
- Conquinine
- Pitayine
- Chinidin
- Conchinin
- (8R,9S)-Quinidine
- (9S)-6'-Methoxycinchonan-9-ol
- beta-Quinine
- Quinidex
- Kinidin
- Conchinine
- Cin-Quin
- beta-Quinidine
- chinidinum
- quinidina
- Quinicardine
- Quinora
- Cinchonan-9-ol, 6'-methoxy-, (9S)-
- Chinidine
- (S)-[(2R,4S,5R)-5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl](6-methoxyquinolin-4-yl)methanol
- CHEBI:28593
- alpha-(6-Methoxy-4-quinolyl)-5-vinyl-2-quinuclidinemethanol
- .beta.-Quinidine
- UNII-ITX08688JL
- Quinaglute
- CCRIS 672
- Quiniduran
- Auriquin
- HSDB 225
- ITX08688JL
- DTXSID4023549
- Quinidine sulfate
- NCI-C56246
- EINECS 200-279-0
- MFCD00135581
- 6-Methoxy-alpha-(5-vinyl-2-quinuclidinyl)-4-quinolinemethanol
- CHEMBL1294
- Quinact
- (1S)-(6-Methoxyquinolin-4-yl)((2R,4S,5R)-5-vinylquinuclidin-2-yl)methanol
- DTXCID70819883
- TCMDC-131239
- Quinidine (>85%)
- alpha-(6-Methoxy-4-quinolyl)-5-vinyl-2-quinuclidinemethanol (9S)-
- (9S)-6-Methoxy-alpha-(5-vinyl-2-quinuclidinyl)-4-quinolinemethanol
- (S)-(6-methoxyquinolin-4-yl)((2R,5R)-5-vinylquinuclidin-2-yl)methanol
- (3'.alpha., 9S)-6'-Methoxycinchonan-9-ol
- QUINIDINE (MART.)
- QUINIDINE [MART.]
- (R)-(6-methoxyquinolin-4-yl)((3S,4R,7S)-3-vinylquinuclidin-7-yl)methanol
- (S)-(6-methoxy-4-quinolyl)-[(2R,4S,5R)-5-vinylquinuclidin-2-yl]methanol
- (S)-(6-Methoxy-quinolin-4-yl)-((2R,5R)-5-vinyl-1-aza-bicyclo[2.2.2]oct-2-yl)-methanol
- (S)-(6-methoxyquinolin-4-yl)((1S,2R,4S,5R)-5-vinylquinuclidin-2-yl)methanol
- (S)-[(4S,5R,7R)-5-ethenyl-1-azabicyclo[2.2.2]octan-7-yl]-(6-methoxyquinolin-4-yl)methanol
- CAS-56-54-2
- Chinidin [German]
- (S)-((2S,4S,5R)-5-ETHENYL-1-AZABICYCLO(2.2.2)OCT-2-YL)(6-METHOXYQUINOLIN-4-YL)METHANOL
- SMR000857275
- Quinidine [BAN:NF]
- Quinidine (may contain up to 15% of dihydroquinidine)
- Quinindine
- (8R,9S)-6'-Methoxycinchonan-9-ol
- (S)-(6-Methoxy-quinolin-4-yl)-((2R,5R)-5-vinyl-1-aza-bicyclo(2.2.2)oct-2-yl)-methanol
- (S)-[(2R,4S,5R)-5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl]-(6-methoxyquinolin-4-yl)methanol
- Quinidine anhydrous
- NCGC00159499-02
- QDN
- Quinidine, anhydrous
- NSC10004
- QUINIDINE [MI]
- QUINIDINE [HSDB]
- Prestwick3_000280
- QUINIDINE [VANDF]
- bmse000511
- Epitope ID:141803
- QUINIDINE [WHO-DD]
- SCHEMBL15943
- BSPBio_000160
- MLS001335913
- MLS001335914
- MLS002548869
- BPBio1_000176
- GTPL2342
- SCHEMBL17537608
- HY-B1751H
- HY-B1751R
- LOUPRKONTZGTKE-LHHVKLHASA-N
- HMS2234L10
- HMS3259O09
- NCI-C56426
- HY-B1751
- Tox21_111720
- Tox21_201110
- 6-methoxy--(5-vinyl-2-quinuclidinyl)-4-quinolinemethanol
- BDBM50121975
- AKOS015920101
- Tox21_111720_1
- CCG-256507
- CS-7812
- DB00908
- NC00478
- SDCCGMLS-0066600.P001
- NCGC00091231-01
- NCGC00091231-02
- NCGC00091231-03
- NCGC00091231-04
- NCGC00091231-18
- NCGC00091231-29
- NCGC00258662-01
- AS-30538
- DA-77296
- QUININE SULFATE IMPURITY A [WHO-IP]
- AB00514657
- CS-1053369
- NS00098768
- Quinidine (15% dihydroquinidine) (Standard)
- QUININE BISULFATE IMPURITY A [WHO-IP]
- EN300-305202
- AB01562940_01
- QUININE SULFATE IMPURITY A [EP IMPURITY]
- AE-508/21131014
- CINCHONAN-9-0L, 6'-METHOXY-, (95)-
- Q412496
- W-109256
- BRD-K59632282-052-01-5
- BRD-K59632282-052-02-3
- BRD-K59632282-052-03-1
- BRD-K70799801-311-02-7
- QUININE HYDROCHLORIDE IMPURITY A [EP IMPURITY]
- Quinidine, crystallized, >=98.0% (dried material, NT)
- Z1741976976
- QUININE BISULFATE HEPTAHYDRATE IMPURITY A [WHO-IP]
- 6'-METHOXY-ALPHA-(5-VINYL-2-QUINUCLIDINYL)-4-QUINOLINEMETHONOL
117.056435 32136
160.074936 31228
146.059097 20872
174.054581 16876
145.051208 14104
325.188812 10320
172.073563 5164
160.074005 4960
307.179169 4804
184.074554 2572
325.1911 999
326.1942 199
327.197 23
325.1916 999
326.1947 196
307.1808 39
327.1976 23
325.19519818339353 100
160.0753603589196 33.18
307.1827504925839 22.52
81.06962240397715 17.11
172.07460727803436 16.12
Quinidine Gluconate (has salt form)
Quinidine Sulfate (active moiety of)
Quinidine bisulfate (is active moiety of)
Quinidine hydrochloride (is active moiety of)
Quinidine hydrochloride monohydrate (is active moiety of)
Quinidine lactate (is active moiety of)
Quinidine dihydrochloride (is active moiety of)
Quinidine polygalacturonate (is active moiety of)
- Extracellular
- Membrane
Use (kg; approx.) in Germany (2009): >500
Use (kg) in USA (2002): 56800
Consumption (g per capita; approx.) in Germany (2009): 0.00611
Consumption (g per capita) in the USA (2002): 0.201
Calculated removal (%): 41.8


H301 (98%): Toxic if swallowed [Danger Acute toxicity, oral]
H315 (10%): Causes skin irritation [Warning Skin corrosion/irritation]
H319 (10%): Causes serious eye irritation [Warning Serious eye damage/eye irritation]
H335 (10%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation]
P261, P264, P264+P265, P270, P271, P280, P301+P316, P302+P352, P304+P340, P305+P351+P338, P319, P321, P330, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 50 reports by companies from 5 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Acute Tox. 3 (98%)
Skin Irrit. 2 (10%)
Eye Irrit. 2A (10%)
STOT SE 3 (10%)
SYMPTOMS: Symptoms of exposure to this compound may include anorexia, nausea, vomiting, abdominal cramps, diarrhea, tinnitus, impaired hearing, blurred vision, vertigo, severe emotional reactions, pyrexia, skin eruptions, thrombocytopenic purpura, asthma, urticaria, acute hemolytic anemia, heart block, diminished cardiac output, ventricular tachycardia, ventricular fibrillation or acute ventricular asystole, headache, confusion, skin rash and angioedema.
ACUTE/CHRONIC HAZARDS: This compound can cause irritation of the skin, eyes and mucous membranes. When heated to decomposition it emits toxic fumes. (NTP, 1992)
EYES: First check the victim for contact lenses and remove if present. Flush victim's eyes with water or normal saline solution for 20 to 30 minutes while simultaneously calling a hospital or poison control center. Do not put any ointments, oils, or medication in the victim's eyes without specific instructions from a physician. IMMEDIATELY transport the victim after flushing eyes to a hospital even if no symptoms (such as redness or irritation) develop.
SKIN: IMMEDIATELY flood affected skin with water while removing and isolating all contaminated clothing. Gently wash all affected skin areas thoroughly with soap and water. If symptoms such as redness or irritation develop, IMMEDIATELY call a physician and be prepared to transport the victim to a hospital for treatment.
INHALATION: IMMEDIATELY leave the contaminated area; take deep breaths of fresh air. If symptoms (such as wheezing, coughing, shortness of breath, or burning in the mouth, throat, or chest) develop, call a physician and be prepared to transport the victim to a hospital. Provide proper respiratory protection to rescuers entering an unknown atmosphere. Whenever possible, Self-Contained Breathing Apparatus (SCBA) should be used; if not available, use a level of protection greater than or equal to that advised under Protective Clothing.
INGESTION: DO NOT INDUCE VOMITING. If the victim is conscious and not convulsing, give 1 or 2 glasses of water to dilute the chemical and IMMEDIATELY call a hospital or poison control center. Be prepared to transport the victim to a hospital if advised by a physician. If the victim is convulsing or unconscious, do not give anything by mouth, ensure that the victim's airway is open and lay the victim on his/her side with the head lower than the body. DO NOT INDUCE VOMITING. IMMEDIATELY transport the victim to a hospital. (NTP, 1992)
Excerpt from ERG Guide 154 [Substances - Toxic and/or Corrosive (Non-Combustible)]:
IMMEDIATE PRECAUTIONARY MEASURE: Isolate spill or leak area in all directions for at least 50 meters (150 feet) for liquids and at least 25 meters (75 feet) for solids.
SPILL: Increase the immediate precautionary measure distance, in the downwind direction, as necessary.
FIRE: If tank, rail tank car or highway tank is involved in a fire, ISOLATE for 800 meters (1/2 mile) in all directions; also, consider initial evacuation for 800 meters (1/2 mile) in all directions. (ERG, 2024)
SMALL SPILLS AND LEAKAGE: If a spill of this chemical occurs, FIRST REMOVE ALL SOURCES OF IGNITION, then you should dampen the solid spill material with acetone and transfer the dampened material to a suitable container. Use absorbent paper dampened with acetone to pick up any remaining material. Seal your contaminated clothing and the absorbent paper in a vapor-tight plastic bag for eventual disposal. Solvent wash all contaminated surfaces with acetone followed by washing with a soap and water solution. Do not reenter the contaminated area until the Safety Officer (or other responsible person) has verified that the area has been properly cleaned.
STORAGE PRECAUTIONS: You should store this material in a refrigerator. (NTP, 1992)
Alcohols and Polyols
Amines, Phosphines, and Pyridines
Hydrocarbons, Aliphatic Unsaturated
IMAP assessments - Cinchonan-9-ol, 6'-methoxy-, (9S)-: Environment tier I assessment
IMAP assessments - Cinchonan-9-ol, 6'-methoxy-, (9S)-: Human health tier I assessment
Chronic therapy with quinidine is associated with a low rate of serum enzyme elevations, which are usually mild, asymptomatic and self limited even without alteration in dose. In addition, there have been many reports of acute hypersensitivity reactions to quinidine that include hepatic involvement. The reactions usually arise after 1 to 2 weeks of therapy, but can appear within 24 hours of restarting quinidine or with rechallenge. The clinical features are marked by fatigue, nausea, vomiting, diffuse muscle aches, arthralgias and high fever. Blood testing at an early stage shows increases in serum aminotransferase and alkaline phosphatase levels as well as mild jaundice, which can deepen for a few days even after stopping quinidine. The pattern of serum enzymes elevations is typically cholestatic or mixed. Rash is uncommon and eosinophilia is not typical, despite the presence of other signs of hypersensitivity (fever, arthralgias). Autoantibodies are not typically found. Liver biopsies usually show mild injury and small epithelioid granulomas, as are often found in many organs during systemic hypersensitivity reactions. A similar clinical signature of liver injury occurs with quinine, an optical isomer of quinidine that is used predominantly as an antimalarial agent. In recent years, there have been few reports of liver injury attributed to quinidine, probably because it is now rarely used.
Likelihood score: A (well established cause of clinically apparent liver injury).
M Chen, V Vijay, Q Shi, Z Liu, H Fang, W Tong. FDA-Approved Drug Labeling for the Study of Drug-Induced Liver Injury, Drug Discovery Today, 16(15-16):697-703, 2011. PMID:21624500 DOI:10.1016/j.drudis.2011.05.007
M Chen, A Suzuki, S Thakkar, K Yu, C Hu, W Tong. DILIrank: the largest reference drug list ranked by the risk for developing drug-induced liver injury in humans. Drug Discov Today 2016, 21(4): 648-653. PMID:26948801 DOI:10.1016/j.drudis.2016.02.015
◉ Summary of Use during Lactation
Limited information indicates that maternal doses of quinidine up to 1.8 grams daily produce low levels in milk and would not be expected to cause any adverse effects in breastfed infants, especially if the infant is older than 2 months. Exclusively breastfed infants should be carefully monitored if this drug is used during lactation, possibly including measurement of serum levels to rule out toxicity if there is a concern.
◉ Effects in Breastfed Infants
Relevant published information was not found as of the revision date.
◉ Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
Occupational hepatotoxin - Secondary hepatotoxins: the potential for toxic effect in the occupational setting is based on cases of poisoning by human ingestion or animal experimentation.
Skin Sensitizer - An agent that can induce an allergic reaction in the skin.
Silke Matysik, Caroline Ivanne Le Roy, Gerhard Liebisch, Sandrine Paule Claus. Metabolomics of fecal samples: A practical consideration. Trends in Food Science & Technology. Vol. 57, Part B, Nov. 2016, p.244-255: http://www.sciencedirect.com/science/article/pii/S0924224416301984
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=LOUPRKONTZGTKE-LHHVKLHASA-N
- Exercise caution with grapefruit products. Grapefruit may delay the absorption of quinidine, and inhibit its metabolism through CYP3A4.
- Exercise caution with St. John's Wort.
- Take separate from antacids. Antacids may reduce the absorption of quinidine.
- Take with or without food. Taking quinidine with food may slow its absorption.
- Australian Industrial Chemicals Introduction Scheme (AICIS)Cinchonan-9-ol, 6'-methoxy-, (9S)-https://services.industrialchemicals.gov.au/search-assessments/
- CAMEO ChemicalsLICENSECAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data.https://cameochemicals.noaa.gov/help/reference/terms_and_conditions.htm?d_f=falseCAMEO Chemical Reactivity Classificationhttps://cameochemicals.noaa.gov/browse/react
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_use
- EPA Chemicals under the TSCACinchonan-9-ol, 6'-methoxy-, (9S)-https://www.epa.gov/chemicals-under-tscaEPA TSCA Classificationhttps://www.epa.gov/tsca-inventory
- EPA DSSToxCompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeQuinidine (EC: 200-279-0)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/13106
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Hazardous Substances Data Bank (HSDB)QUINIDINE SULFATEhttps://pubchem.ncbi.nlm.nih.gov/source/hsdb/5515
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingHMDB0015044_msms_374799https://hmdb.ca/metabolites/HMDB0015044#spectra
- BindingDBLICENSEAll data curated by BindingDB staff are provided under the Creative Commons Attribution 3.0 License (https://creativecommons.org/licenses/by/3.0/us/).https://www.bindingdb.org/rwd/bind/info.jsp(6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2.2.2]oct-2-yl)-methanolhttps://www.bindingdb.org/rwd/bind/chemsearch/marvin/MolStructure.jsp?monomerid=50121975
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jsp
- Drug Gene Interaction database (DGIdb)LICENSEThe data used in DGIdb is all open access and where possible made available as raw data dumps in the downloads section.http://www.dgidb.org/downloads
- IUPHAR/BPS Guide to PHARMACOLOGYLICENSEThe Guide to PHARMACOLOGY database is licensed under the Open Data Commons Open Database License (ODbL) https://opendatacommons.org/licenses/odbl/. Its contents are licensed under a Creative Commons Attribution-ShareAlike 4.0 International License (http://creativecommons.org/licenses/by-sa/4.0/)https://www.guidetopharmacology.org/about.jsp#licenseGuide to Pharmacology Target Classificationhttps://www.guidetopharmacology.org/targets.jsp
- Burnham Center for Chemical Genomics
- Haz-Map, Information on Hazardous Chemicals and Occupational DiseasesLICENSECopyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://haz-map.com/AboutQuinidinehttps://haz-map.com/Agents/1346
- ChEBI
- FDA Pharm ClassesLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingFDA Pharmacological Classificationhttps://www.fda.gov/ForIndustry/DataStandards/StructuredProductLabeling/ucm162549.htm
- LiverTox
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/LOTUS Treehttps://lotus.naturalproducts.net/
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licence
- CCSbaseCCSbase Classificationhttps://ccsbase.net/
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- IUPAC Digitized pKa Dataset
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- Crystallography Open Database (COD)LICENSEAll data in the COD and the database itself are dedicated to the public domain and licensed under the CC0 License. Users of the data should acknowledge the original authors of the structural data.https://creativecommons.org/publicdomain/zero/1.0/
- DailyMed
- Drug Induced Liver Injury Rank (DILIrank) DatasetLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/QuinidineNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- Drugs and Lactation Database (LactMed)
- Drugs@FDALICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- EU Clinical Trials Register
- FDA Orange BookLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- National Drug Code (NDC) DirectoryLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.html
- Natural Product Activity and Species Source (NPASS)
- The Natural Products AtlasLICENSEThe Natural Products Atlas is licensed under a Creative Commons Attribution 4.0 International License.https://www.npatlas.org/termsThe Natural Products Atlas Classificationhttps://www.npatlas.org/
- MassBank Europe
- Metabolomics Workbench
- Nature Chemistry
- NIPH Clinical Trials Search of Japan
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.htmlquinidine sulfatehttps://rxnav.nlm.nih.gov/id/rxnorm/9069
- PharmGKBLICENSEPharmGKB data are subject to the Creative Commons Attribution-ShareALike 4.0 license (https://creativecommons.org/licenses/by-sa/4.0/).https://www.pharmgkb.org/page/policies
- PharosLICENSEData accessed from Pharos and TCRD is publicly available from the primary sources listed above. Please respect their individual licenses regarding proper use and redistribution.https://pharos.nih.gov/about
- Protein Data Bank in Europe (PDBe)
- RCSB Protein Data Bank (RCSB PDB)LICENSEData files contained in the PDB archive (ftp://ftp.wwpdb.org) are free of all copyright restrictions and made fully and freely available for both non-commercial and commercial use. Users of the data should attribute the original authors of that structural data.https://www.rcsb.org/pages/policies
- SpectraBaseEPIQUINIDINEhttps://spectrabase.com/spectrum/JGsL1604o3V
- Springer Nature
- SpringerMaterials
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- WHO Anatomical Therapeutic Chemical (ATC) ClassificationLICENSEUse of all or parts of the material requires reference to the WHO Collaborating Centre for Drug Statistics Methodology. Copying and distribution for commercial purposes is not allowed. Changing or manipulating the material is not allowed.https://www.whocc.no/copyright_disclaimer/
- Wikidata
- WikipediaBlepharismahttps://en.wikipedia.org/wiki/Blepharisma
- Wiley
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlAdrenergic alpha-Antagonistshttps://www.ncbi.nlm.nih.gov/mesh/68000317Muscarinic Antagonistshttps://www.ncbi.nlm.nih.gov/mesh/68018727Anti-Arrhythmia Agentshttps://www.ncbi.nlm.nih.gov/mesh/68000889Cytochrome P-450 CYP2D6 Inhibitorshttps://www.ncbi.nlm.nih.gov/mesh/68065690Antimalarialshttps://www.ncbi.nlm.nih.gov/mesh/68000962Enzyme Inhibitorshttps://www.ncbi.nlm.nih.gov/mesh/68004791Voltage-Gated Sodium Channel Blockershttps://www.ncbi.nlm.nih.gov/mesh/68061567
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403392621https://pubchem.ncbi.nlm.nih.gov/substance/403392621
- NCBI






