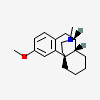Levomethorphan
- Levomethorphan
- l-Methorphan
- Methorphan
- Levomethorphane
- Levometorfano
- Create:2004-09-16
- Modify:2025-01-18
 Dextromethorphan (annotation moved to).
Dextromethorphan (annotation moved to).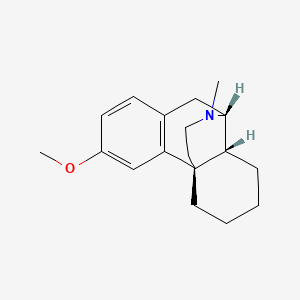
- l-methorphan
- levomethorphan
- Levomethorphan
- l-Methorphan
- Methorphan
- Levomethorphane
- Levometorfano
- 125-70-2
- Levomethorphanum
- (-)-3-Methoxy-N-methylmorphinan
- Levometorfano [INN-Spanish]
- Levomethorphane [INN-French]
- Levomethorphanum [INN-Latin]
- RACEMETHORPHAN
- Racemethorphanum
- Morphinan, 3-methoxy-17-methyl-, l-
- Racemethorphane
- Racemetorfano
- 7ZZ22K9QE6
- 8YB8F78WM1
- IDS-NL-004
- IDS-NL-005
- Levomethorphan (INN)
- Dextromethorfan [Czech]
- Destrometerfano [DCIT]
- LEVOMETHORPHAN [INN]
- Dextrometorphan
- 510-53-2
- Dextromethorphan-d3
- Tylenol Cough + Decongestant Liquid
- Demorphine
- Dimacol
- Deoxydihydrothebacodine
- Rondec dm
- Robitussin CF
- Robitussin DM
- (1R,9R,10R)-4-methoxy-17-methyl-17-azatetracyclo[7.5.3.01,10.02,7]heptadeca-2(7),3,5-triene
- Levomethorphan (1.0mg/ml in Acetonitrile)
- Levomethorphan [INN:BAN:DCF]
- Prestwick_686
- Benylin DM (TN)
- Demorphan hydrobromide
- EINECS 204-751-7
- IDS-NR-001
- DL-3-Methoxy-N-methylmorphinan
- UNII-7ZZ22K9QE6
- UNII-8YB8F78WM1
- 3-Methoxy-17-methylmorphinan, l
- Racemetorfano [INN-Spanish]
- Racemethorphane [INN-French]
- Racemethorphanum [INN-Latin]
- Dexyromethorphan
- ((-))-3-Methoxy-N-methylmorphinan
- DEA No. 9210
- Levomethorphan [BAN:DCF:INN]
- Racemethorphan [INN:BAN:DCF]
- 2H-10,4a-Iminoethanophenanthrene, 1,3,4,9,10,10a-hexahydro-6-methoxy-11-methyl-
- Morphinan, 3-methoxy-N-methyl-, (-)-
- EINECS 208-114-4
- Morphinan, 3-methoxy-17-methyl-, (-)-
- (+-)-3-methoxy-17-methylmorphinan
- Dextromethorphan Bromhydrate
- LEVOMETHORPHAN [MI]
- RACEMETHORPHAN [MI]
- RACEMETHORPHAN [INN]
- SCHEMBL29950
- DEA No. 9732
- CHEMBL1908323
- L-3-methoxy-17-methylmorphinan
- DTXSID20872403
- DTXSID60872402
- CHEBI:146176
- Morphinan, 3-methoxy-17-methyl-
- (+)-3-Methoxy-9a-methylmorphinan
- (+-)-3-Methoxy-N-methylmorphinan
- (-)-3-methoxy-17-methylmorphinan
- 524713-56-2
- NSC751452
- (+/-)-3-methoxy-17-methylmorphinan
- AKOS015969688
- (9alpha,13alpha,14alpha)-3-Methoxy-17-methylmorphinan hydrobromide
- dl-cis-1,3,4,9,10,10a-hexahydro-6-methoxy-11-methyl-2H-10,4a-iminoethanophenanthrene
- Dextromethorphan hydrobromide [BAN:JAN]
- AC-13098
- (+/-)-3-METHOXY-N-METHYLMORPHINAN
- NS00068001
- NS00068002
- D12696
- W-108400
- Q60998690
- Levomethorphan, vial of 10 mg, certified reference material
- (9S,13S,14S)-6,18-Dideoxy-7,8-dihydro-3-O-methylmorphine
- (4aR,10R,10aR)-6-methoxy-11-methyl-1,3,4,9,10,10a-hexahydro-2H-10,4a-(epiminoethano)phenanthrene
- DL-CIS-1,2,3,9,10,10A-HEXAHYDRO-6-METHOXY-11-METHYL-4H-10,4A-IMINOETHANOPHENANTHRENE
59.0 99.99
271.0 38
150.0 34.40
214.0 20.30
31.0 19.70
59 99.99
271 38
150 34.40
214 20.30
31 19.70
279.253 100
195.291 23.94
276.872 21.62
176.245 21.04
358.469 19.88
80.904 100
82.282 91.99
212.708 54.60
181.382 28.44
232.132 22.02
 Dextromethorphan (annotation moved to)
Dextromethorphan (annotation moved to)
P264, P270, P301+P317, P330, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 39 reports by companies from 2 notifications to the ECHA C&L Inventory.
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
◉ Summary of Use during Lactation
The amounts of dextromethorphan and its active metabolite in breastmilk are very low and are not expected to affect the nursing infant. It is best to avoid the use of products with a high alcohol content while nursing.
◉ Effects in Breastfed Infants
Relevant published information was not found as of the revision date.
◉ Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=MKXZASYAUGDDCJ-CGTJXYLNSA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/Levomethorphanhttps://commonchemistry.cas.org/detail?cas_rn=125-70-2(±)-3-Methoxy-17-methylmorphinanhttps://commonchemistry.cas.org/detail?cas_rn=510-53-2
- ChemIDplusLevomethorphan [INN:BAN:DCF]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000125702Racemethorphan [INN:BAN:DCF]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000510532ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DTP/NCILICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuse
- EPA DSSToxRacemethorphanhttps://comptox.epa.gov/dashboard/DTXSID60872402Levomethorphanhttps://comptox.epa.gov/dashboard/DTXSID20872403CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-notice[No public or meaningful name is available]https://echa.europa.eu/substance-information/-/substanceinfo/100.230.710Levomethorphan (EC: 204-751-7)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/40759
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingDextromethorphanhttp://www.hmdb.ca/metabolites/HMDB0001920HMDB0001920_cms_27714https://hmdb.ca/metabolites/HMDB0001920#spectra
- ChEBI
- Drug Enforcement Administration (DEA)LICENSEUnless otherwise indicated, information on Department of Justice websites is in the public domain and may be copied and distributed without permission. Citation of the Department of Justice as source of the information is appreciated, as appropriate.https://www.justice.gov/legalpoliciesLevomethorphanhttps://www.deadiversion.usdoj.gov/schedules/DEA drug and chemical classificationhttps://www.dea.gov/drug-information/drug-scheduling
- Toxin and Toxin Target Database (T3DB)LICENSET3DB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (T3DB) and the original publication.http://www.t3db.ca/downloadsDextromethorphanhttp://www.t3db.ca/toxins/T3D2815
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- Drugs and Lactation Database (LactMed)Dextromethorphanhttps://www.ncbi.nlm.nih.gov/books/n/lactmed/LM572/
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- SpectraBaseDextromethorphanhttps://spectrabase.com/spectrum/CVlFhpF3fOSDextromethorphanhttps://spectrabase.com/spectrum/8Gk0gnBs5MuDextromethorphanehttps://spectrabase.com/spectrum/H3GvNf2aSvD
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlTarget-based classification of drugshttp://www.genome.jp/kegg-bin/get_htext?br08310.keg
- Metabolomics Workbench
- Natural Product Activity and Species Source (NPASS)
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawLevomethorphanhttp://www.nist.gov/srd/nist1a.cfm
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/RacemethorphanNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- Springer Nature
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidatalevomethorphanhttps://www.wikidata.org/wiki/Q60998690
- WikipediaLevomethorphanhttps://en.wikipedia.org/wiki/LevomethorphanMethorphanhttps://en.wikipedia.org/wiki/Methorphan
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmllevomethorphanhttps://www.ncbi.nlm.nih.gov/mesh/2107154Analgesics, Opioidhttps://www.ncbi.nlm.nih.gov/mesh/68000701
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403560324https://pubchem.ncbi.nlm.nih.gov/substance/403560324
- NCBI