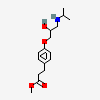
Esmolol
- ESMOLOL
- 81147-92-4
- 103598-03-4
- (+-)-Esmolol
- ASL 8052-001
- Create:2005-06-24
- Modify:2025-01-18
 Esmolol Hydrochloride (has salt form).
Esmolol Hydrochloride (has salt form).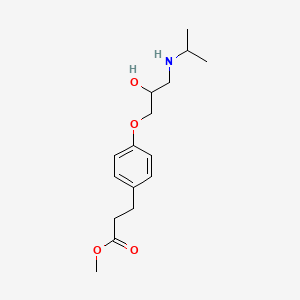
- ASL 8052
- ASL-8052
- Brevibloc
- esmolol
- esmolol hydrochloride
- ESMOLOL
- 81147-92-4
- 103598-03-4
- (+-)-Esmolol
- ASL 8052-001
- (+/-)-esmolol
- UNII-MDY902UXSR
- MDY902UXSR
- methyl 3-[4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl]propanoate
- Esmolol (INN)
- Benzenepropanoic acid, 4-(2-hydroxy-3-((1-methylethyl)amino)propoxy)-, methyl ester
- BRN 5287174
- Methyl 4-(2-hydroxy-3-((1-methylethyl)amino)propoxy)benzenepropanoate
- ASL-8052-001
- DTXSID4022995
- CHEBI:88206
- SL-8052
- methyl p-(2-hydroxy-3-(isopropylamino)propoxy)hydrocinnamate
- ESMOLOL [INN]
- (+/-)-methyl p-(2-hydroxy-3-(isopropylamino)propoxy)hydrocinnamate
- Esmolol [INN:BAN]
- methyl 3-{4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl}propanoate
- methyl 3-(4-{2-hydroxy-3-[(propan-2-yl)amino]propoxy}phenyl)propanoate
- 3-(4-(2-HYDROXY-3-(ISOPROPYLAMINO)PROPOXY)PHENYL)PROPIONIC ACID METHYL ESTER
- BENZENEPROPANOIC ACID, 4-(2-HYDROXY-3-((1-METHYLETHYL)AMINO)PROPOXY)-, METHYL ESTER, (+/-)-
- NCGC00185766-01
- esmololum
- SR-01000763706
- 3-(4-(2-Hydroxy-3-isopropylamino-propoxy)-phenyl)-propionic acid methyl ester
- 3-[4-(2-Hydroxy-3-isopropylamino-propoxy)-phenyl]-propionic acid methyl ester
- 3-[4-[2-Hydroxy-3-(isopropylamino)propoxy]phenyl]propionic Acid Methyl Ester
- Benzenepropanoic acid, 4-[2-hydroxy-3-[(1-methylethyl)amino]propoxy]-, methyl ester
- ESMOLOL [VANDF]
- ESMOLOL [MI]
- methyl 3-(4-(2-hydroxy-3-(isopropylamino)propoxy)phenyl)propanoate
- ESMOLOL [WHO-DD]
- CHEMBL768
- SCHEMBL3605
- CHEBI:4856
- DTXCID702995
- GTPL7178
- HY-B1392A
- C07AB09
- HMS2090P06
- HMS3743M15
- HMS3886F03
- (+-)-methyl p-(2-hydroxy-3-(isopropylamino)propoxy)hydrocinnamate
- BDBM50404796
- Methyl 3-[4-[2-Hydroxy-3-(isopropylamino)propoxy]phenyl]propionate
- MFCD00864566
- s5778
- AKOS015960734
- methyl 3-[4-({2-hydroxy-3-[(1-methylethyl)amino]propyl}oxy)phenyl]propanoate
- DB00187
- MRF-0000253
- NCGC00185766-02
- NCGC00185766-05
- AC-12058
- AC-35197
- DB-015362
- DB-297892
- E1106
- NS00002313
- C06980
- D07916
- D90594
- AB00698516-07
- AB00698516-09
- AB00698516_10
- EN300-7384765
- L001332
- Q418139
- SR-01000763706-3
- BRD-A07395371-003-10-0
- rac-methyl 3-(4-(2-hydroxy-3-(propan-2-ylamino)propoxy)phenyl)propanoate
296.18927 100
297.190369 50.79
145.065689 36.79
219.102142 13.58
133.065323 7.04
296.18857 100
145.06563 62.74
219.1049 38.23
116.10717 18.27
98.09773 14.13
105 999
115 960
103 787
118 646
107 627
145 999
133 772
105 603
119 537
107 419
 Esmolol Hydrochloride (has salt form)
Esmolol Hydrochloride (has salt form)Use (kg; approx.) in Germany (2009): >10
Use (kg) in USA (2002): 423
Consumption (g per capita; approx.) in Germany (2009): 0.000122
Consumption (g per capita) in the USA (2002): 0.0015
Calculated removal (%): 46.6

H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation]
H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation]
H335 (100%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation]
P261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Esmolol therapy has not been clearly associated with serum aminotransferase elevations or with clinically apparent, acute liver injury. It is often used in critically patients and generally for a short period only. Thus, hepatotoxicity due to esmolol must be very rare, if it occurs at all. Most commonly used beta-blockers have been linked to rare instances of clinically apparent liver injury, typically with onset within 2 to 12 weeks, a hepatocellular pattern of liver enzyme elevations, rapid recovery upon withdrawal, and little evidence of hypersensitivity (rash, fever, eosinophilia) or autoantibody formation. Similar instances have not been reported after esmolol use.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
M Chen, V Vijay, Q Shi, Z Liu, H Fang, W Tong. FDA-Approved Drug Labeling for the Study of Drug-Induced Liver Injury, Drug Discovery Today, 16(15-16):697-703, 2011. PMID:21624500 DOI:10.1016/j.drudis.2011.05.007
M Chen, A Suzuki, S Thakkar, K Yu, C Hu, W Tong. DILIrank: the largest reference drug list ranked by the risk for developing drug-induced liver injury in humans. Drug Discov Today 2016, 21(4): 648-653. PMID:26948801 DOI:10.1016/j.drudis.2016.02.015
◉ Summary of Use during Lactation
No published information is available on the use esmolol during breastfeeding. Based on its physicochemical properties and extremely short half-life, esmolol would not be expected to cause any adverse effects in breastfed infants.
◉ Effects in Breastfed Infants
A study of mothers taking beta-blockers during nursing found a numerically, but not statistically significant increased number of adverse reactions in those taking any beta-blocker. Although the ages of infants were matched to control infants, the ages of the affected infants were not stated. None of the mothers were taking esmolol.
◉ Effects on Lactation and Breastmilk
Relevant published information on the effects of beta-blockade or esmolol during normal lactation was not found as of the revision date. A study in 6 patients with hyperprolactinemia and galactorrhea found no changes in serum prolactin levels following beta-adrenergic blockade with propranolol.
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=AQNDDEOPVVGCPG-UHFFFAOYSA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_use
- EPA DSSToxCompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeMethyl 3[4-[2-Hydroxy-3-(Isopropylamino)Propoxy]Phenyl] Propionatehttps://echa.europa.eu/substance-information/-/substanceinfo/100.157.881Methyl 3[4-[2-Hydroxy-3-(Isopropylamino)Propoxy]Phenyl] Propionate (EC: 629-713-0)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/162597
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingHMDB0014333_msms_450987https://hmdb.ca/metabolites/HMDB0014333#spectra
- ChEBIMethyl 3-{4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl}propanoatehttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:88206
- FDA Pharm ClassesLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingFDA Pharmacological Classificationhttps://www.fda.gov/ForIndustry/DataStandards/StructuredProductLabeling/ucm162549.htm
- LiverTox
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licence
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- DailyMed
- Drug Gene Interaction database (DGIdb)LICENSEThe data used in DGIdb is all open access and where possible made available as raw data dumps in the downloads section.http://www.dgidb.org/downloads
- IUPHAR/BPS Guide to PHARMACOLOGYLICENSEThe Guide to PHARMACOLOGY database is licensed under the Open Data Commons Open Database License (ODbL) https://opendatacommons.org/licenses/odbl/. Its contents are licensed under a Creative Commons Attribution-ShareAlike 4.0 International License (http://creativecommons.org/licenses/by-sa/4.0/)https://www.guidetopharmacology.org/about.jsp#licenseGuide to Pharmacology Target Classificationhttps://www.guidetopharmacology.org/targets.jsp
- Therapeutic Target Database (TTD)
- Drug Induced Liver Injury Rank (DILIrank) DatasetLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Drugs and Lactation Database (LactMed)
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/ESMOLOLNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- EU Clinical Trials Register
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-law
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlAnatomical Therapeutic Chemical (ATC) classificationhttp://www.genome.jp/kegg-bin/get_htext?br08303.kegTarget-based classification of drugshttp://www.genome.jp/kegg-bin/get_htext?br08310.keg
- MassBank Europe
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- Metabolomics Workbench
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- NIPH Clinical Trials Search of Japan
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.html
- WHO Anatomical Therapeutic Chemical (ATC) ClassificationLICENSEUse of all or parts of the material requires reference to the WHO Collaborating Centre for Drug Statistics Methodology. Copying and distribution for commercial purposes is not allowed. Changing or manipulating the material is not allowed.https://www.whocc.no/copyright_disclaimer/
- PharmGKBLICENSEPharmGKB data are subject to the Creative Commons Attribution-ShareALike 4.0 license (https://creativecommons.org/licenses/by-sa/4.0/).https://www.pharmgkb.org/page/policies
- PharosLICENSEData accessed from Pharos and TCRD is publicly available from the primary sources listed above. Please respect their individual licenses regarding proper use and redistribution.https://pharos.nih.gov/about
- SpectraBase
- Springer Nature
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidata
- WikipediaArsenic trisulfidehttps://en.wikipedia.org/wiki/Arsenic_trisulfide
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlAdrenergic beta-1 Receptor Antagonistshttps://www.ncbi.nlm.nih.gov/mesh/68058671
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 388588882https://pubchem.ncbi.nlm.nih.gov/substance/388588882
- NCBI