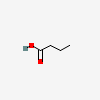
Butyric Acid
- C4H8O2
- CH3CH2CH2COOH
- butyric acid
- butanoic acid
- 107-92-6
- n-Butyric acid
- n-Butanoic acid
- Create:2004-09-16
- Modify:2025-01-25
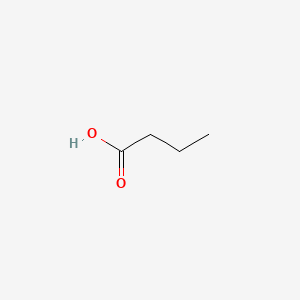
C4H8O2
CH3CH2CH2COOH
- Acid, Butanoic
- Acid, Butyric
- Butanoic Acid
- Butyrate, Magnesium
- Butyrate, Sodium
- Butyric Acid
- Butyric Acid Magnesium Salt
- Butyric Acid, Sodium Salt
- Dibutyrate, Magnesium
- Magnesium Butyrate
- Magnesium Dibutyrate
- Sodium Butyrate
- butyric acid
- butanoic acid
- 107-92-6
- n-Butyric acid
- n-Butanoic acid
- propylformic acid
- ethylacetic acid
- 1-propanecarboxylic acid
- butyrate
- Butanic acid
- 1-Butyric acid
- Buttersaeure
- butanoate
- Butyric acid (natural)
- Kyselina maselna
- FEMA No. 2221
- Propanecarboxylic acid
- 1-butanoic acid
- acide butyrique
- butoic acid
- Buttersaeure [German]
- Butyricum acidum
- FEMA Number 2221
- CCRIS 6552
- HSDB 940
- Kyselina maselna [Czech]
- NSC 8415
- fattyacids
- MFCD00002814
- acide butanoique
- 2-butanoate
- NORMAL BUTYRIC ACID
- AI3-15306
- NSC-8415
- EINECS 203-532-3
- UNII-40UIR9Q29H
- UN2820
- BRN 0906770
- 40UIR9Q29H
- C4:0
- DTXSID8021515
- CHEBI:30772
- Acid, Butanoic
- NSC8415
- BUTYRIC ACID (D8)
- CH3-[CH2]2-COOH
- DTXCID401515
- EC 203-532-3
- butanate
- 4-02-00-00779 (Beilstein Handbook Reference)
- n-butanoate
- 1-butanoate
- propanecarboxylate
- 1-butyrate
- Butyric acid [UN2820] [Corrosive]
- Sodium n-butyrate
- 1-propanecarboxylate
- BUA
- CH3-(CH2)2-COOH
- Butyric Acid (Normal)
- Acid, Butyric
- CAS-107-92-6
- sodium-butyrate
- Honey robber
- Tetranoic Acid
- ethyl acetic acid
- 1ugp
- 3umq
- butanoic acid, 4
- TNFa + NaBut
- 715 - Chocolate
- Butyrate, sodium salt
- Butyric acid [UN2820] [Corrosive]
- BUTYRIC_ACID
- n-C3H7COOH
- TNFa + Sodium Butyrate
- Butyric acid, >=99%
- bmse000402
- BUTYRIC ACID [MI]
- NCIMech_000707
- BUTYRIC ACID [FCC]
- WLN: QV3
- BUTYRIC ACID [HSDB]
- BUTYRIC ACID [VANDF]
- CHEMBL14227
- BUTYRIC ACID [WHO-DD]
- Butyric acid, >=99%, FG
- N-BUTYRIC ACID [FHFI]
- GTPL1059
- BUTYRICUM ACIDUM [HPUS]
- BDBM26109
- Butyric acid, analytical standard
- Bio1_000444
- Bio1_000933
- Bio1_001422
- STR06290
- Tox21_202382
- Tox21_300164
- CCG-35836
- FA 4:0
- LMFA01010004
- STL169349
- AKOS000118961
- DB03568
- UN 2820
- NCGC00247914-01
- NCGC00247914-02
- NCGC00247914-05
- NCGC00253919-01
- NCGC00259931-01
- BP-21420
- NCI60_001424
- 1ST1702-1000W
- Butyric acid, natural, >=99%, FCC, FG
- DB-318878
- B0754
- Butyric acid Solution in Water, 1000?g/mL
- NS00001284
- Butyric acid 1000 microg/mL in Acetonitrile
- Butyric acid, SAJ special grade, >=99.5%
- EN300-21334
- C00246
- Q193213
- W-108732
- BRD-K05878375-236-02-4
- BRD-K05878375-236-05-7
- BRD-K05878375-236-06-5
- 9B27B3D0-9643-40EC-9A5F-7CA1A6ED7F9F
- F2191-0094
- Z104495380
- InChI=1/C4H8O2/c1-2-3-4(5)6/h2-3H2,1H3,(H,5,6
- Boiling point
- Chemical diffusion
- Chemical shift
- Corrosion
- Crystal structure
- Density
- Diamagnetic susceptibility
- Dielectric constant
- Diffusion
- Diffusive flux
- Excess enthalpy
- Formula unit
- Fusion temperature
- Heat capacity
- Heat of solution
- Heat of sublimation
- Lineshape
- Magnetic susceptibility
- Melting temperature
- Mixing enthalpy
- Optical coefficient
- Phase diagram
- Phase equilibrium
- Phase transition
- Refractive index
- Sound absorption
- Sound propagation
- Sound velocity
- Space group
- Surface tension
- Thermal expansion coefficient
- Transition enthalpy
- Unit cell
- Unit cell parameter
- Vapor pressure
- Vapor-liquid equilibrium
- Viscosity
60.0 99.99
73.0 28.50
27.0 23.48
41.0 22.36
42.0 18.70
60.0 99.99
27.0 32.60
73.0 30.27
41.0 24.41
42.0 21.65
60.0 1
73.0 0.29
27.0 0.24
41.0 0.22
42.0 0.19
60.0 1
27.0 0.33
73.0 0.30
41.0 0.24
42.0 0.22
87.1 999
43.1 1
57.9 1
42.2 1
104.9 1
87.3 999
42.9 4
44 4
69.2 2
58.3 1
60 999
73 285
27 235
41 224
42 187
- Fibroblasts
- Intestine
- Kidney
- Neuron
- Prostate
- Skeletal Muscle
- Cytoplasm
- Extracellular
- Membrane
- Mitochondria
Painting (Pigments, Binders, and Biocides) [Category: Paint]
Leather Tanning and Processing [Category: Industry]
Fur Dressing and Dyeing [Category: Industry]
- Fragrance
- Intermediate
- Processing aids, not otherwise listed
2019: 20,000,000 lb - <100,000,000 lb
2018: 20,000,000 lb - <100,000,000 lb
2017: 20,000,000 lb - <100,000,000 lb
2016: 20,000,000 lb - <100,000,000 lb
- All Other Chemical Product and Preparation Manufacturing
- All Other Basic Organic Chemical Manufacturing


H302 (12.7%): Harmful if swallowed [Warning Acute toxicity, oral]
H314 (99%): Causes severe skin burns and eye damage [Danger Skin corrosion/irritation]
H318 (11.3%): Causes serious eye damage [Danger Serious eye damage/eye irritation]
P260, P264, P264+P265, P270, P280, P301+P317, P301+P330+P331, P302+P361+P354, P304+P340, P305+P354+P338, P316, P317, P321, P330, P363, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 2102 reports by companies from 14 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Acute Tox. 4 (12.7%)
Skin Corr. 1B (99%)
Eye Dam. 1 (11.3%)

· TOXIC and/or CORROSIVE; inhalation, ingestion or skin contact with material may cause severe injury or death.
· Methyl bromoacetate (UN2643) is an eye irritant/lachrymator (causes flow of tears).
· Contact with molten substance may cause severe burns to skin and eyes.
· Avoid any skin contact.
· Fire may produce irritating, corrosive and/or toxic gases.
· Runoff from fire control or dilution water may be corrosive and/or toxic and cause environmental contamination.
Excerpt from ERG Guide 153 [Substances - Toxic and/or Corrosive (Combustible)]:
Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Those substances designated with a (P) may polymerize explosively when heated or involved in a fire. Corrosives in contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form. (ERG, 2024)
· Combustible material: may burn but does not ignite readily.
· When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards.
· Those substances designated with a (P) may polymerize explosively when heated or involved in a fire.
· Corrosives in contact with metals may evolve flammable hydrogen gas.
· Containers may explode when heated.
· Runoff may pollute waterways.
· Substance may be transported in a molten form.
EYES: First check the victim for contact lenses and remove if present. Flush victim's eyes with water or normal saline solution for 20 to 30 minutes while simultaneously calling a hospital or poison control center. Do not put any ointments, oils, or medication in the victim's eyes without specific instructions from a physician. IMMEDIATELY transport the victim after flushing eyes to a hospital even if no symptoms (such as redness or irritation) develop.
SKIN: IMMEDIATELY flood affected skin with water while removing and isolating all contaminated clothing. Gently wash all affected skin areas thoroughly with soap and water. IMMEDIATELY call a hospital or poison control center even if no symptoms (such as redness or irritation) develop. IMMEDIATELY transport the victim to a hospital for treatment after washing the affected areas.
INHALATION: IMMEDIATELY leave the contaminated area; take deep breaths of fresh air. If symptoms (such as wheezing, coughing, shortness of breath, or burning in the mouth, throat, or chest) develop, call a physician and be prepared to transport the victim to a hospital. Provide proper respiratory protection to rescuers entering an unknown atmosphere. Whenever possible, Self-Contained Breathing Apparatus (SCBA) should be used; if not available, use a level of protection greater than or equal to that advised under Protective Clothing.
INGESTION: DO NOT INDUCE VOMITING. Corrosive chemicals will destroy the membranes of the mouth, throat, and esophagus and, in addition, have a high risk of being aspirated into the victim's lungs during vomiting which increases the medical problems. If the victim is conscious and not convulsing, give 1 or 2 glasses of water to dilute the chemical and IMMEDIATELY call a hospital or poison control center. IMMEDIATELY transport the victim to a hospital. If the victim is convulsing or unconscious, do not give anything by mouth, ensure that the victim's airway is open and lay the victim on his/her side with the head lower than the body. DO NOT INDUCE VOMITING. Transport the victim IMMEDIATELY to a hospital. (NTP, 1992)
General First Aid:
· Call 911 or emergency medical service.
· Ensure that medical personnel are aware of the material(s) involved, take precautions to protect themselves and avoid contamination.
· Move victim to fresh air if it can be done safely.
· Administer oxygen if breathing is difficult.
· If victim is not breathing:
-- DO NOT perform mouth-to-mouth resuscitation; the victim may have ingestedor inhaled the substance.
-- If equipped and pulse detected, wash face and mouth, then give artificial respiration using a proper respiratory medical device (bag-valve mask, pocket mask equipped with a one-way valve or other device).
-- If no pulse detected or no respiratory medical device available, provide continuouscompressions. Conduct a pulse check every two minutes or monitor for any signs of spontaneous respirations.
· Remove and isolate contaminated clothing and shoes.
· For minor skin contact, avoid spreading material on unaffected skin.
· In case of contact with substance, remove immediately by flushing skin or eyes with running water for at least 20 minutes.
· For severe burns, immediate medical attention is required.
· Effects of exposure (inhalation, ingestion, or skin contact) to substance may be delayed.
· Keep victim calm and warm.
· Keep victim under observation.
· For further assistance, contact your local Poison Control Center.
· Note: Basic Life Support (BLS) and Advanced Life Support (ALS) should be done by trained professionals.
Specific First Aid:
· For corrosives, in case of contact, immediately flush skin or eyes with running water for at least 30 minutes. Additional flushing may be required.
· Removal of solidified molten material from skin requires medical assistance.
In Canada, an Emergency Response Assistance Plan (ERAP) may be required for this product. Please consult the shipping paper and/or the "ERAP" section.
Excerpt from ERG Guide 153 [Substances - Toxic and/or Corrosive (Combustible)]:
SMALL FIRE: Dry chemical, CO2 or water spray.
LARGE FIRE: Dry chemical, CO2, alcohol-resistant foam or water spray. If it can be done safely, move undamaged containers away from the area around the fire. Dike runoff from fire control for later disposal.
FIRE INVOLVING TANKS, RAIL TANK CARS OR HIGHWAY TANKS: Fight fire from maximum distance or use unmanned master stream devices or monitor nozzles. Do not get water inside containers. Cool containers with flooding quantities of water until well after fire is out. Withdraw immediately in case of rising sound from venting safety devices or discoloration of tank. ALWAYS stay away from tanks in direct contact with flames. (ERG, 2024)
· CALL 911. Then call emergency response telephone number on shipping paper. If shipping paper not available or no answer, refer to appropriate telephone number listed on the inside back cover.
· Keep unauthorized personnel away.
· Stay upwind, uphill and/or upstream.
· Ventilate closed spaces before entering, but only if properly trained and equipped.
· ELIMINATE all ignition sources (no smoking, flares, sparks or flames) from immediate area.
· Do not touch damaged containers or spilled material unless wearing appropriate protective clothing.
· Stop leak if you can do it without risk.
· Prevent entry into waterways, sewers, basements or confined areas.
· Absorb or cover with dry earth, sand or other non-combustible material and transfer to containers.
· DO NOT GET WATER INSIDE CONTAINERS.
Excerpt from ERG Guide 153 [Substances - Toxic and/or Corrosive (Combustible)]:
IMMEDIATE PRECAUTIONARY MEASURE: Isolate spill or leak area in all directions for at least 50 meters (150 feet) for liquids and at least 25 meters (75 feet) for solids.
SPILL: Increase the immediate precautionary measure distance, in the downwind direction, as necessary.
FIRE: If tank, rail tank car or highway tank is involved in a fire, ISOLATE for 800 meters (1/2 mile) in all directions; also, consider initial evacuation for 800 meters (1/2 mile) in all directions. (ERG, 2024)
Immediate precautionary measure
· Isolate spill or leak area in all directions for at least 50 meters (150 feet) for liquids and at least 25 meters (75 feet) for solids.
Spill
· For highlighted materials: see Table 1 - Initial Isolation and Protective Action Distances.
· For non-highlighted materials: increase the immediate precautionary measure distance, in the downwind direction, as necessary.
Fire
· If tank, rail tank car or highway tank is involved in a fire, ISOLATE for 800 meters (1/2 mile) in all directions; also, consider initial evacuation for 800 meters (1/2 mile) in all directions.
Excerpt from ERG Guide 153 [Substances - Toxic and/or Corrosive (Combustible)]:
ELIMINATE all ignition sources (no smoking, flares, sparks or flames) from immediate area. Do not touch damaged containers or spilled material unless wearing appropriate protective clothing. Stop leak if you can do it without risk. Prevent entry into waterways, sewers, basements or confined areas. Absorb or cover with dry earth, sand or other non-combustible material and transfer to containers. DO NOT GET WATER INSIDE CONTAINERS. (ERG, 2024)
· Wear positive pressure self-contained breathing apparatus (SCBA).
· Wear chemical protective clothing that is specifically recommended by the manufacturer when there is NO RISK OF FIRE.
· Structural firefighters' protective clothing provides thermal protection but only limited chemical protection.
Small Fire
· Dry chemical, CO2 or water spray.
Large Fire
· Dry chemical, CO2, alcohol-resistant foam or water spray.
· If it can be done safely, move undamaged containers away from the area around the fire.
· Dike runoff from fire control for later disposal.
Fire Involving Tanks, Rail Tank Cars or Highway Tanks
· Fight fire from maximum distance or use unmanned master stream devices or monitor nozzles.
· Do not get water inside containers.
· Cool containers with flooding quantities of water until well after fire is out.
· Withdraw immediately in case of rising sound from venting safety devices or discoloration of tank.
· ALWAYS stay away from tanks in direct contact with flames.
IMAP assessments - Butanoic acid: Environment tier I assessment
IMAP assessments - Butanoic acid: Human health tier II assessment
Silke Matysik, Caroline Ivanne Le Roy, Gerhard Liebisch, Sandrine Paule Claus. Metabolomics of fecal samples: A practical consideration. Trends in Food Science & Technology. Vol. 57, Part B, Nov. 2016, p.244-255: http://www.sciencedirect.com/science/article/pii/S0924224416301984
PubMed: 6589104, 16277678, 15338487, 10361015, 15249323
Tie-juan ShaoZhi-xing HeZhi-jun XieHai-chang LiMei-jiao WangCheng-ping Wen. Characterization of ankylosing spondylitis and rheumatoid arthritis using 1H NMR-based metabolomics of human fecal extracts. Metabolomics. April 2016, 12:70: https://link.springer.com/article/10.1007/s11306-016-1000-2
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=FERIUCNNQQJTOY-UHFFFAOYSA-N
- Australian Industrial Chemicals Introduction Scheme (AICIS)
- CAMEO ChemicalsLICENSECAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data.https://cameochemicals.noaa.gov/help/reference/terms_and_conditions.htm?d_f=falseBUTYRIC ACIDhttps://cameochemicals.noaa.gov/chemical/2749CAMEO Chemical Reactivity Classificationhttps://cameochemicals.noaa.gov/browse/react
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_useButyric Acidhttps://www.drugbank.ca/drugs/DB03568
- DTP/NCILICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuse
- EPA Chemical Data Reporting (CDR)LICENSEThe U.S. Government retains a nonexclusive, royalty-free license to publish or reproduce these documents, or allow others to do so, for U.S. Government purposes. These documents may be freely distributed and used for non-commercial, scientific and educational purposes.https://www.epa.gov/web-policies-and-procedures/epa-disclaimers#copyrightButanoic acidhttps://www.epa.gov/chemical-data-reporting
- EPA Chemicals under the TSCAButanoic acidhttps://www.epa.gov/chemicals-under-tscaEPA TSCA Classificationhttps://www.epa.gov/tsca-inventory
- EPA DSSToxButanoic acidhttps://comptox.epa.gov/dashboard/DTXSID8021515CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeButyric acidhttps://chem.echa.europa.eu/100.003.212Butyric acid (EC: 203-532-3)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/132308
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Hazardous Substances Data Bank (HSDB)n-BUTYRIC ACIDhttps://pubchem.ncbi.nlm.nih.gov/source/hsdb/940
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingButyric acidhttp://www.hmdb.ca/metabolites/HMDB0000039HMDB0000039_cms_27166https://hmdb.ca/metabolites/HMDB0000039#spectra
- ILO-WHO International Chemical Safety Cards (ICSCs)
- International Fragrance Association (IFRA)LICENSE(c) The International Fragrance Association, 2007-2021. All rights reserved.https://ifrafragrance.org/links/copyright
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/
- NJDOH RTK Hazardous Substance List
- BindingDBLICENSEAll data curated by BindingDB staff are provided under the Creative Commons Attribution 3.0 License (https://creativecommons.org/licenses/by/3.0/us/).https://www.bindingdb.org/rwd/bind/info.jsp
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jsp
- IUPHAR/BPS Guide to PHARMACOLOGYLICENSEThe Guide to PHARMACOLOGY database is licensed under the Open Data Commons Open Database License (ODbL) https://opendatacommons.org/licenses/odbl/. Its contents are licensed under a Creative Commons Attribution-ShareAlike 4.0 International License (http://creativecommons.org/licenses/by-sa/4.0/)https://www.guidetopharmacology.org/about.jsp#licenseGuide to Pharmacology Target Classificationhttps://www.guidetopharmacology.org/targets.jsp
- Therapeutic Target Database (TTD)Butanoic Acidhttps://idrblab.net/ttd/data/drug/details/D0T4JQ
- Toxin and Toxin Target Database (T3DB)LICENSET3DB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (T3DB) and the original publication.http://www.t3db.ca/downloadsButyric acidhttp://www.t3db.ca/toxins/T3D4592
- Emergency Response Guidebook (ERG)Butyric acidhttps://pubchem.ncbi.nlm.nih.gov/erg/
- Haz-Map, Information on Hazardous Chemicals and Occupational DiseasesLICENSECopyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://haz-map.com/AboutButyric acidhttps://haz-map.com/Agents/1391
- Joint FAO/WHO Expert Committee on Food Additives (JECFA)LICENSEPermission from WHO is not required for the use of WHO materials issued under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Intergovernmental Organization (CC BY-NC-SA 3.0 IGO) licence.https://www.who.int/about/policies/publishing/copyright
- ChEBI
- E. coli Metabolome Database (ECMDB)
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/Butyric Acidhttps://www.wikidata.org/wiki/Q193213LOTUS Treehttps://lotus.naturalproducts.net/
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licenceBUTANOIC ACIDhttps://platform.opentargets.org/drug/CHEMBL14227
- Yeast Metabolome Database (YMDB)butanoic acidhttps://www.ymdb.ca/compounds/YMDB01392
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- Crystallography Open Database (COD)LICENSEAll data in the COD and the database itself are dedicated to the public domain and licensed under the CC0 License. Users of the data should acknowledge the original authors of the structural data.https://creativecommons.org/publicdomain/zero/1.0/
- IUPAC Digitized pKa DatasetButanoic acidhttps://github.com/IUPAC/Dissociation-Constants
- ECI Group, LCSB, University of Luxembourgbutyric acid
- KNApSAcK Species-Metabolite Database
- Natural Product Activity and Species Source (NPASS)
- EPA Chemical and Products Database (CPDat)EPA CPDat Classificationhttps://www.epa.gov/chemical-research/chemical-and-products-database-cpdat
- EU Food Improvement Agents
- Hazardous Chemical Information System (HCIS), Safe Work Australia
- NITE-CMCbutyric acid - FY2008 (New/original classication)https://www.chem-info.nite.go.jp/chem/english/ghs/08-mhlw-0159e.html
- Regulation (EC) No 1272/2008 of the European Parliament and of the CouncilLICENSEThe copyright for the editorial content of this source, the summaries of EU legislation and the consolidated texts, which is owned by the EU, is licensed under the Creative Commons Attribution 4.0 International licence.https://eur-lex.europa.eu/content/legal-notice/legal-notice.htmlbutyric acidhttps://eur-lex.europa.eu/eli/reg/2008/1272/oj
- FDA Substances Added to FoodLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Flavor and Extract Manufacturers Association (FEMA)
- FooDBLICENSEFooDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (FooDB) and the original publication.https://foodb.ca/aboutn-butanoatehttps://foodb.ca/compounds/FDB031031
- NMRShiftDB
- MassBank Europe
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawButanoic acidhttp://www.nist.gov/srd/nist1a.cfm
- SpectraBaseBUTYRIC ACIDhttps://spectrabase.com/spectrum/A2TbVHLwrpNBUTANOIC ACIDhttps://spectrabase.com/spectrum/DAvBHvNMXDwBUTYRIC ACIDhttps://spectrabase.com/spectrum/EXAa2lvWCmlButyric acidhttps://spectrabase.com/spectrum/Hou4zambGEHBUTYRIC ACIDhttps://spectrabase.com/spectrum/DOgtYn5anQOButyric acidhttps://spectrabase.com/spectrum/5GP9TBkBFloBUTYRIC ACIDhttps://spectrabase.com/spectrum/I9R4PGCmIsJButyric acidhttps://spectrabase.com/spectrum/4RM6D8m53oTBUTANOIC-ACID;BUTTERSAEUREhttps://spectrabase.com/spectrum/HSZAGzt27c8N-BUTYRIC_ACIDhttps://spectrabase.com/spectrum/DBkpK0sJoWButyric acidhttps://spectrabase.com/spectrum/IYwiCQRcLDlButyric acidhttps://spectrabase.com/spectrum/4dIqsrxS4J1Butyric acidhttps://spectrabase.com/spectrum/I7rPU4IoPgbButyric acidhttps://spectrabase.com/spectrum/6BWc6PDs2cIButyric acidhttps://spectrabase.com/spectrum/KOoVXkCVBODButanoic acidhttps://spectrabase.com/spectrum/EanfHeWmZqvButanoic acidhttps://spectrabase.com/spectrum/4ynMeOV9U4KButanoic acidhttps://spectrabase.com/spectrum/3uO07gYJLMA
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlCompounds with biological roleshttp://www.genome.jp/kegg-bin/get_htext?br08001.kegPhytochemical compoundshttp://www.genome.jp/kegg-bin/get_htext?br08003.keg
- LIPID MAPSLipid Classificationhttps://www.lipidmaps.org/
- MarkerDBLICENSEThis work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.https://markerdb.ca/Butyric acidhttps://markerdb.ca/chemicals/28
- Metabolomics Workbench
- Nature Chemical Biology
- Nature Chemistry
- NIPH Clinical Trials Search of Japan
- PharosLICENSEData accessed from Pharos and TCRD is publicly available from the primary sources listed above. Please respect their individual licenses regarding proper use and redistribution.https://pharos.nih.gov/aboutbutyric acidhttps://pharos.nih.gov/ligands/BK9YBU47BFRU
- Protein Data Bank in Europe (PDBe)
- RCSB Protein Data Bank (RCSB PDB)LICENSEData files contained in the PDB archive (ftp://ftp.wwpdb.org) are free of all copyright restrictions and made fully and freely available for both non-commercial and commercial use. Users of the data should attribute the original authors of that structural data.https://www.rcsb.org/pages/policies
- Springer Nature
- SpringerMaterials
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidatabutyric acidhttps://www.wikidata.org/wiki/Q193213
- WikipediaButyric acidhttps://en.wikipedia.org/wiki/Butyric_acid
- Wiley
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlButyric Acidhttps://www.ncbi.nlm.nih.gov/mesh/68020148Histamine Antagonistshttps://www.ncbi.nlm.nih.gov/mesh/68006633
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403029659https://pubchem.ncbi.nlm.nih.gov/substance/403029659
- NCBI