1,1-Dichloro-1,2,2,2-tetrafluoroethane
PubChem CID
9775
Molecular Formula
Synonyms
- 1,1-Dichlorotetrafluoroethane
- 1,1-DICHLORO-1,2,2,2-TETRAFLUOROETHANE
- 374-07-2
- Frigen 114A
- CFC-114a
Molecular Weight
170.92 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Dates
- Create:2005-03-27
- Modify:2025-01-04
Chemical Structure Depiction
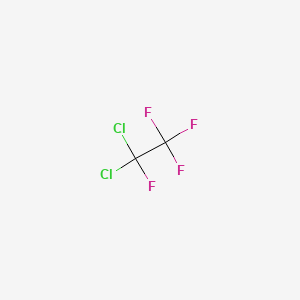
1,1-dichloro-1,2,2,2-tetrafluoroethane
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C2Cl2F4/c3-1(4,5)2(6,7)8
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
BAMUEXIPKSRTBS-UHFFFAOYSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
C(C(F)(Cl)Cl)(F)(F)F
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C2Cl2F4
Computed by PubChem 2.2 (PubChem release 2021.10.14)
374-07-2
- 1,1-Dichlorotetrafluoroethane
- 1,1-DICHLORO-1,2,2,2-TETRAFLUOROETHANE
- 374-07-2
- Frigen 114A
- CFC-114a
- Ethane, 1,1-dichloro-1,2,2,2-tetrafluoro-
- Dichlorotetrafluroethane
- 1320-37-2
- Ethane, 1,1-dichlorotetrafluoro-
- HSDB 5564
- UNII-8AWA8IET6R
- 8AWA8IET6R
- 1,1,1,2-Tetrafluoro-2,2-dichloroethane
- EINECS 206-774-8
- FREON 114A
- FREON-114A
- DTXSID9027150
- R-114A
- 1,1-DICHLORO-1,2,2,2-TETRAFLUOROETHANE [HSDB]
- SCHEMBL866
- 1,1Dichlorotetrafluoroethane
- Ethane, 1,1dichlorotetrafluoro
- DTXCID407150
- 1,1,1,2Tetrafluoro2,2dichloroethane
- Ethane, 1,1dichloro1,2,2,2tetrafluoro
- NS00041744
- Q161277
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
170.92 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
XLogP3
Property Value
2.9
Reference
Computed by XLogP3 3.0 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
0
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
4
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
0
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
169.9313180 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
169.9313180 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
0Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
8
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
84.5
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Liquid
Colorless gas; [MSDSonline]
Colorless
Braker W, Mossman A; Matheson Gas Data Book 6th ED p.251 (1980)
Odorless
Braker W, Mossman A; Matheson Gas Data Book 6th ED p.251 (1980)
3.4 °C
Haynes, W.M. (ed.) CRC Handbook of Chemistry and Physics. 91st ed. Boca Raton, FL: CRC Press Inc., 2010-2011, p. 3-160
-56.6 °C
Haynes, W.M. (ed.) CRC Handbook of Chemistry and Physics. 91st ed. Boca Raton, FL: CRC Press Inc., 2010-2011, p. 3-160
In water,137 mg/L at 25 °C
Hine J, Mookerjee PK; J Org Chem 40: 292-8 (1975)
Haynes, W.M. (ed.) CRC Handbook of Chemistry and Physics. 91st ed. Boca Raton, FL: CRC Press Inc., 2010-2011, p. 3-160
1.455 g/cu cm at 25 °C
Haynes, W.M. (ed.) CRC Handbook of Chemistry and Physics. 91st ed. Boca Raton, FL: CRC Press Inc., 2010-2011, p. 3-160
Liquid density: 1.55 g/mL at 25 °C
Smart BE, Fernandez RE; Kirk-Othmer Encyclopedia of Chemical Technology. (1999-2012). New York, NY: John Wiley & Sons; Fluorinated Aliphatic Compounds. Online Posting Date: December 4, 2000
1640 mm Hg at 25 °C
Daubert, T.E., R.P. Danner. Physical and Thermodynamic Properties of Pure Chemicals Data Compilation. Washington, D.C.: Taylor and Francis, 1989.
Under certain conditions, /chlorofluorocarbon/ vapors may decompose on contact with flames or hot surfaces, creating the potential hazard of inhalation of toxic decomposition products. /Chlorofluorocarbon/
Clayton, G.D., F.E. Clayton (eds.) Patty's Industrial Hygiene and Toxicology. Volumes 2A, 2B, 2C, 2D, 2E, 2F: Toxicology. 4th ed. New York, NY: John Wiley & Sons Inc., 1993-1994., p. 1195
The appearance of toxic decomposition products serves as warning of the occurrence of thermal decompositon and detection of a sharp acrid odor warns of the presence of these products. /Fluorocarbons/
Clayton, G. D. and F. E. Clayton (eds.). Patty's Industrial Hygiene and Toxicology: Volume 2A, 2B, 2C: Toxicology. 3rd ed. New York: John Wiley Sons, 1981-1982., p. 3101
1.9924X10-3 Pa.s at 216.58 K
Daubert, T.E., R.P. Danner. Physical and Thermodynamic Properties of Pure Chemicals Data Compilation. Washington, D.C.: Taylor and Francis, 1989.
2.6156X10+7 J/kmol at 216.58 K
Daubert, T.E., R.P. Danner. Physical and Thermodynamic Properties of Pure Chemicals Data Compilation. Washington, D.C.: Taylor and Francis, 1989.
2.7790X10-2 N/m at 216.58 K
Daubert, T.E., R.P. Danner. Physical and Thermodynamic Properties of Pure Chemicals Data Compilation. Washington, D.C.: Taylor and Francis, 1989.
Index of refraction: 1.3092 at 0 °C/D
U.S. Department of Health & Human Services/National Toxicology Program; 10th Report on Carcinogens, Research Triangle Park, NC 2002, p. 3-160
Ozone Depletion Potential = 1
Environmental Defense Fund; Scorecard. Report for 1,1-Dichloro- 1,2,2,2-tetrafluoroethane (374-07-2). Available from, as of Decl 19, 2012: https://www.scorecard.org/
Solvents -> Chlorofluorocarbons
1D NMR Spectra
NIST Number
35022
Library
Main library
Total Peaks
60
m/z Top Peak
135
m/z 2nd Highest
101
m/z 3rd Highest
85
Thumbnail
NIST Number
6722
Library
Replicate library
Total Peaks
62
m/z Top Peak
135
m/z 2nd Highest
101
m/z 3rd Highest
85
Thumbnail
Other MS
MASS: 25033 (NIST/EPA/MSDC Mass Spectral Database, 1990 version); 1116 (Atlas of Mass Spectral Data, John Wiley & Sons, New York)
IR Spectra
IR: 2075 (Documentation of Molecular Spectroscopy Collection)
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
PubMed Count
... Main factor affecting fate of fluorocarbons is body fat, where they are concentrated & slowly released into blood at concn that should not cause any risk of cardiac sensitization.
National Research Council. Drinking Water & Health Volume 1. Washington, DC: National Academy Press, 1977., p. 781
There is a significant accumulation of fluorocarbons in brain, liver and lung compared to blood levels, signifying a tissue distribution of fluorocarbons similar to that of chloroform.
Clayton, G.D., F.E. Clayton (eds.) Patty's Industrial Hygiene and Toxicology. Volumes 2A, 2B, 2C, 2D, 2E, 2F: Toxicology. 4th ed. New York, NY: John Wiley & Sons Inc., 1993-1994., p. 1203
Regardless of the route of entry, chlorofluorocarbons appear to be eliminated almost exclusively through the respiratory tract. Little, if any, chlorofluorocarbon or metabolite has ever been reported in urine or feces. /Chlorofluorocarbons/
WHO; Environmental Health Criteria 113: Fully Halogenated Chlorofluorocarbons p.60 (1990)
Sources/Uses
Can be used as a refrigerant, solvent, and aerosol propellant; No report of commercial use in the US; [HSDB]
Aerosol propellant, refrigerant, solvent, fire extinguisher, blowing agent, and dielectric fluid /CFC 114/
Rusch GM; Patty's Toxicology. (1999-2012). New York, NY: John Wiley & Sons, In Organic Chlorofluoro Hydrocarbons. On-line posting date: Aug 17, 2012
Intermediates
The most important commercial method for manufacturing /chlorofluoro carbons/ is the successive replacement of chlorine by fluorine using hydrogen fluoride. The liquid-phase process uses antimony pentafluoride or a mixture of antimony trifluoride and chlorine as catalysts. Continuous vapor-phase processes employ gaseous hydrogen fluoride in the presence of heterogenous chromium, iron or fluorinated alumina catalysts.
Smart BE, Fernandez RE; Kirk-Othmer Encyclopedia of Chemical Technology. (1999-2012). New York, NY: John Wiley & Sons; Fluorinated Aliphatic Compounds. Online Posting Date: December 4, 2000
Commercial manufacture of the closely related isomer CClF2CClF2, is achieved by the direct chlorination of tetrafluoroethylene
The most important commercial method for manufacturing CFCs and HCFCs is the successive replacement of chlorine by fluorine using hydrogen fluoride. The traditional, liquid-phase process uses antimony pentafluoride or a mixture of antimony trifluoride and chlorine as catalysts. Continuous vapor-phase processes that employ gaseous hydrogen fluoride in the presence of heterogenous chromium, iron, or fluorinated alumina catalysts also are widely used. Carbon tetrachloride, chloroform, and hexachloroethane (or tetrachloroethylene plus chlorine) are commonly used starting materials for one- and two-carbon chlorofluorocarbons. The extent of chlorine exchange can be controlled by varying the hydrogen fluoride concentration, the contact time, or the reaction temperature. /CFCs and HCFCs/
Kirk-Othmer Encyclopedia of Chemical Technology. 4th ed. Volumes 1: New York, NY. John Wiley and Sons, 1991-Present., p. V11 507 (1994)
Chlorofluoroalkanes (and also the alternative HCFCs and HFCs) produced on an industrial scale are subject to stringent standards. Impurities must not exceed the following limits (vol %): acids, 0; moisture, <0.001; higher-boiling fractions, <0.05; and other gases, 2. /Chlorofluoroalkanes/
Siegemund G et al; Ullmann's Encyclopedia of Industrial Chemistry 7th ed. (1999-2013). NY, NY: John Wiley & Sons; Fluorine Compounds, Organic. Online Posting Date: June 15, 2000
Aggregated Product Volume
2019: 20,000,000 lb - <100,000,000 lb
2018: 100,000,000 lb - <1,000,000,000 lb
2017: 100,000,000 lb - <1,000,000,000 lb
2016: 100,000,000 lb - <1,000,000,000 lb
(1977) AT LEAST 4.54X10+8 G
SRI
(1981) AT LEAST 6.81X10+6 G-UNSPECIFIED ISOMER
SRI
Ethane, 1,1-dichloro-1,2,2,2-tetrafluoro- is listed as a High Production Volume (HPV) chemical (65FR81686). Chemicals listed as HPV were produced in or imported into the U.S. in >1 million pounds in 1990 and/or 1994. The HPV list is based on the 1990 Inventory Update Rule. (IUR) (40 CFR part 710 subpart B; 51FR21438).
EPA/Office of Pollution Prevention and Toxics; High Production Volume (HPV) Challenge Program. Ethane, 1,1-dichloro-1,2,2,2-tetrafluoro- (374-07-2). Available from, as of February 28, 2013: https://www.epa.gov/hpv/pubs/general/opptsrch.htm
Production volume for non-confidential chemicals reported under the 2006 Inventory Update Rule. Chemical: Ethane, 1,1-dichloro-1,2,2,2-tetrafluoro-. Aggregated National Production Volume: 100 to < 500 million lbs.
US EPA; Non-Confidential 2006 Inventory Update Reporting. National Chemical Information. Ethane, 1,1-dichloro-1,2,2,2-tetrafluoro- (374-07-2). Available from, as of February 28, 2013: https://cfpub.epa.gov/iursearch/index.cfm?s=chem&err=t
Non-confidential 2012 Chemical Data Reporting (CDR) information on the production and use of chemicals manufactured or imported into the United States. Chemical: Ethane, 1,1-dichloro-1,2,2,2-tetrafluoro-. National Production Volume: withheld.
USEPA/Pollution Prevention and Toxics; 2012 Chemical Data Reporting Database. Ethane, 1,1-dichloro-1,2,2,2-tetrafluoro- (374-07-2). Available from, as of February 28, 2013: https://java.epa.gov/oppt_chemical_search/
Industry Processing Sectors
Industrial Gas Manufacturing
EPA TSCA Commercial Activity Status
Ethane, 1,1-dichloro-1,2,2,2-tetrafluoro-: ACTIVE
SRP: The EPA has organized groups of chemicals into two classes according to their ozone-depletion potential. Class I controlled substances are those with an ozone-depletion potential of 0.2 or higher. Class II controlled substances are those with an ozone-potential of less than 0.2. Class II controlled substances are all hydrochlorofluorocarbons (HCFCs).
In the United States, "Class I" substances were subject to the first round of phaseout targets. Class I substances have an ozone depletion potential (ODP) of 0.2 or higher, and include halons, chlorofluorocarbons (CFCs), methyl chloroform, carbon tetrachloride, and methyl bromide. Section 604 of the Clean Air Act sets the phaseout targets for Class I substances. The ban on production and import of halons took effect on January 1, 1994. The ban on production and import of other Class I ODS /ozone-depleting substance/ - excluding methyl bromide - took effect on January 1, 1996.
USEPA; Ozone Layer Protection-Regulatory Programs. Phaseout of Class I Ozone-Depleting Substances. Available from, as of March 16, 2013: https://www.epa.gov/ozone/title6/phaseout/classone.html
Class I Controlled Substance: C2F4Cl2 - Dichlorotetrafluoroethane (CFC-114) /and all isomers/: Ozone-depletion potential: 1.0.
40 CFR Appendix A to Subpart A of Part 82 (USEPA); U.S. National Archives and Records Administration's Electronic Code of Federal Regulations. Available from, as of March 16, 2013: https://www.ecfr.gov/cgi-bin/ECFR?page=browse
... /The use of chlorofluorocarbons/ for aerosol sprays was prohibited as of 1979, except for a few specialized items, because of their depleting effect on stratospheric ozone. /Chlorofluorocarbons/
Lewis, R.J. Sr.; Hawley's Condensed Chemical Dictionary 15th Edition. John Wiley & Sons, Inc. New York, NY 2007., p. 281
For more General Manufacturing Information (Complete) data for 1,1-DICHLORO-1,2,2,2-TETRAFLUOROETHANE (6 total), please visit the HSDB record page.
Fluorocarbons in air of working area & in exhaled air can be analyzed by IR spectrometry. /Fluorocarbons/
TRIEBIG G, BURKHARDT K; INT ARCH OCCUP ENVIRON HEALTH 42 (2): 129-36 (1979)
This paper dicusses /use of an/ electron capture detector to analyze atmospheric chlorofluorocarbons and ways of improving its accuracy. /Chlorofluorocarbons/
LOVELOCK JE, WATSON J; J CHROMATOGR (158): 123-38 (1978)
Gas chromatographic method for determination of fluorocarbons in air is described. /Fluorocarbons/
RAUWS ET AL; J PHARM PHARMACOL 25 (9): 718-22 (1973)
Gas chromatographic method for measuring halocarbons in ambient air samples is presented. /Halocarbons/
LILLIAN ET AL; J ENVIRON SCI HEALTH A-11 (12): 687-710 (1976)
Gas chromatographic method for analysis of fluorocarbons in body fluids is described. /Fluorocarbons/
RAUWS ET AL; J PHARM PHARMACOL 25 (9): 718-22 (1973)
Hexane extraction procedure for the determination of common fluorocarbon propellants in blood was evaluated. An analysis of sample headspace was also evaluated for determining chloropentafluoroethane in blood. Both procedures involved analysis by gas chromatography using electron capture detection. The widely used hexane extraction procedure for determining ppm levels of volatile halocarbons in tissue was evaluated by a combination of radiochemical and gas chromatographic techniques. The data suggest that hexane extraction gives significantly low results. /Fluorocarbons/
TERRILL JB; AMER IND HYG ASSOC J 33 (11): 736-44 (1972)
May cause irritation; Inhalation of high concentrations can cause CNS depression; [MSDSonline] See CHLOROFLUOROCARBONS.
Non-flammable gas /1,2-Dichloro-1,1,2,2-Tetrafluoroethane/
Association of American Railroads; Bureau of Explosives. Emergency Handling of Hazardous Materials in Surface Transportation. Association of American Railroads, Pueblo, CO. 2005, p. 313
Critical temperature: 145.6 °C; critical pressure: 3.29 MPa
Smart BE, Fernandez RE; Kirk-Othmer Encyclopedia of Chemical Technology. (1999-2012). New York, NY: John Wiley & Sons; Fluorinated Aliphatic Compounds. Online Posting Date: December 4, 2000
If material on fire or involved in fire: Extinguish fire using agent suitable for type of surrounding fire. (Material itself does not burn or burns with difficulty). Cool all affected containers with flooding quantities of water. Apply water from as far a distance as possible. Do not use water on material itself. Use water spray to knock-down vapors. /1,2-Dichloro-1,1,2,2-Tetrafluoroethane/
Association of American Railroads; Bureau of Explosives. Emergency Handling of Hazardous Materials in Surface Transportation. Association of American Railroads, Pueblo, CO. 2005, p. 313
Evacuation: If fire becomes uncontrollable or container is exposed to direct flame - consider evacuation of one-half (1/2) mile radius. /1,2-Dichloro-1,1,2,2-Tetrafluoroethane/
Association of American Railroads; Bureau of Explosives. Emergency Handling of Hazardous Materials in Surface Transportation. Association of American Railroads, Pueblo, CO. 2005, p. 313
SRP: The most favorable course of action is to use an alternative chemical product with less inherent propensity for occupational harm/injury/toxicity or environmental contamination. Recycle any unused portion of the material for its approved use or return it to the manufacturer or supplier. Ultimate disposal of the chemical must consider: the material's impact on air quality; potential migration in soil or water; effects on animal and plant life; and conformance with environmental and public health regulations.
Dichlorotetrafluoroethane is a waste chemical stream constituent which may be subjected to ultimate disposal by controlled incineration. Incineration is done, preferably after mixing with another combustible fuel. Care must be exercised to assure complete combustion to prevent the formation of phosgene. An acid scrubber is necessary to remove the halo acids produced.
USEPA; Engineering Handbook for Hazardous Waste Incineration p.2-5 (1981) EPA 68-03-3025
Personnel protection: Keep upwind. Avoid breathing vapors. ... Avoid bodily contact with the material. /1,2-Dichloro-1,1,2,2-Tetrafluoroethane/
Association of American Railroads; Bureau of Explosives. Emergency Handling of Hazardous Materials in Surface Transportation. Association of American Railroads, Pueblo, CO. 2005, p. 313
If material not involved in fire: Attempt to stop leak if without undue personnel hazard. Use water spray to knock-down vapors. /1,2-Dichloro-1,1,2,2-Tetrafluoroethane/
Association of American Railroads; Bureau of Explosives. Emergency Handling of Hazardous Materials in Surface Transportation. Association of American Railroads, Pueblo, CO. 2005, p. 313
SRP: Contaminated protective clothing should be segregated in such a manner so that there is no direct personal contact by personnel who handle, dispose, or clean the clothing. Quality assurance to ascertain the completeness of the cleaning procedures should be implemented before the decontaminated protective clothing is returned for reuse by the workers. Contaminated clothing should not be taken home at end of shift, but should remain at employee's place of work for cleaning.
Inhalation of vapors should be avoided. /Chlorofluorocarbon/
Clayton, G.D., F.E. Clayton (eds.) Patty's Industrial Hygiene and Toxicology. Volumes 2A, 2B, 2C, 2D, 2E, 2F: Toxicology. 4th ed. New York, NY: John Wiley & Sons Inc., 1993-1994., p. 1195
For more Preventive Measures (Complete) data for 1,1-DICHLORO-1,2,2,2-TETRAFLUOROETHANE (8 total), please visit the HSDB record page.
The Montreal Protocol on Substances that Deplete the Ozone Layer was designed to reduce the production and consumption of ozone depleting substances in order to reduce their abundance in the atmosphere, and thereby protect the earth's fragile ozone Layer. The original Montreal Protocol was agreed on 16 September 1987 and entered into force on 1 January 1989. The Montreal Protocol includes a unique adjustment provision that enables the Parties to the Protocol to respond quickly to new scientific information and agree to accelerate the reductions required on chemicals already covered by the Protocol. These adjustments are then automatically applicable to all countries that ratified the Protocol. Since its initial adoption, the Montreal Protocol has been adjusted five times. Specifically, the Second, Fourth, Seventh, Ninth, Eleventh and Nineteenth Meetings of the Parties to the Montreal Protocol adopted, in accordance with the procedure laid down in paragraph 9 of Article 2 of the Montreal Protocol, certain adjustments and reductions of production and consumption of the controlled substances listed in the Annexes of the Protocol. These adjustments entered into force, for all the Parties, on 7 March 1991, 23 September 1993, 5 August 1996, 4 June 1998, 28 July 2000 and 14 May 2008, respectively. In addition to adjusting the Protocol, the Parties to the Montreal Protocol have amended the Protocol to enable, among other things, the control of new chemicals and the creation of a financial mechanism to enable developing countries to comply. Specifically, the Second, Fourth, Ninth and Eleventh Meetings of the Parties to the Montreal Protocol adopted, in accordance with the procedure laid down in paragraph 4 of Article 9 of the Vienna Convention, four Amendments to the Protocol - the London Amendment (1990), the Copenhagen Amendment (1992), the Montreal Amendment (1997) and the Beijing Amendment (1999). Unlike adjustments to the Protocol, amendments must be ratified by countries before their requirements are applicable to those countries. The London, Copenhagen, Montreal and Beijing Amendments entered into force on 10 August 1992, 14 June 1994, 10 November 1999 and 25 February 2002, respectively, only for those Parties which ratified the particular amendments. In addition to adjustments and amendments to the Montreal Protocol, the Parties to the Protocol meet annually and take a variety of decisions aimed at enabling effective implementation of this important legal instrument. Through the 22nd Meeting of the Parties to the Montreal Protocol, the Parties have taken over 720 decisions.
United Nations Environment Programme; Ozone Secretariat - The Montreal Protocol on Substances that Deplete the Ozone Layer. Available from, as of May 30, 2013: https://ozone.unep.org/new_site/en/montreal_protocol.php
Forced air ventilation and level of vapor concentration together with the use of individual breathing devices with independent air supply will minimize risk of inhalation. Lifelines should be worn when entering tanks or other confined spaces. /Chlorofluorocarbon/
Clayton, G.D., F.E. Clayton (eds.) Patty's Industrial Hygiene and Toxicology. Volumes 2A, 2B, 2C, 2D, 2E, 2F: Toxicology. 4th ed. New York, NY: John Wiley & Sons Inc., 1993-1994., p. 1195
Neoprene gloves, protective clothing, and eye protection minimize risk of topical contact. /Chlorofluorocarbon or Hydrochlorofluorocarbon/
Clayton, G.D., F.E. Clayton (eds.) Patty's Industrial Hygiene and Toxicology. Volumes 2A, 2B, 2C, 2D, 2E, 2F: Toxicology. 4th ed. New York, NY: John Wiley & Sons Inc., 1993-1994., p. 1198
/GUIDE 126: GASES - COMPRESSED OR LIQUEFIED (INCLUDING REFRIGERANT GASES)/ Fire or Explosion: Some may burn, but none ignite readily. Containers may explode when heated. Ruptured cylinders may rocket.
U.S. Department of Transportation. 2008 Emergency Response Guidebook. Washington, D.C. 2008
/GUIDE 126: GASES - COMPRESSED OR LIQUEFIED (INCLUDING REFRIGERANT GASES)/ Health: Vapors may cause dizziness or asphyxiation without warning. Vapors from liquefied gas are initially heavier than air and spread along ground. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire may produce irritating, corrosive and/or toxic gases.
U.S. Department of Transportation. 2008 Emergency Response Guidebook. Washington, D.C. 2008
/GUIDE 126: GASES - COMPRESSED OR LIQUEFIED (INCLUDING REFRIGERANT GASES)/ Public Safety: CALL Emergency Response Telephone Number ... As an immediate precautionary measure, isolate spill or leak area for at least 100 meters (330 feet) in all directions. Keep unauthorized personnel away. Stay upwind. Many gases are heavier than air and will spread along ground and collect in low or confined areas (sewers, basements, tanks). Keep out of low areas. Ventilate closed spaces before entering.
U.S. Department of Transportation. 2008 Emergency Response Guidebook. Washington, D.C. 2008
/GUIDE 126: GASES - COMPRESSED OR LIQUEFIED (INCLUDING REFRIGERANT GASES)/ Protective Clothing: Wear positive pressure self-contained breathing apparatus (SCBA). Wear chemical protective clothing that is specifically recommended by the manufacturer. It may provide little or no thermal protection. Structural firefighters' protective clothing will only provide limited protection.
U.S. Department of Transportation. 2008 Emergency Response Guidebook. Washington, D.C. 2008
For more DOT Emergency Guidelines (Complete) data for 1,1-DICHLORO-1,2,2,2-TETRAFLUOROETHANE (8 total), please visit the HSDB record page.
49 045 33; Refrigerant gas, not otherwise specified or dispersant gas not otherwise specified (gas mixtures, not elsewhere classified, compressed, other than poison)
The Australian Inventory of Industrial Chemicals
Chemical: Ethane, 1,1-dichloro-1,2,2,2-tetrafluoro-
The Food and Drug Administration (FDA), after consultation with the Environmental Protection Agency (EPA), is amending FDA's regulation on the use of ozone-depleting substances (ODSs) in selfpressurized containers to remove the essential-use designations for flunisolide, triamcinolone, metaproterenol, pirbuterol, albuterol and ipratropium in combination, cromolyn, and nedocromil used in oral pressurized metered-dose inhalers (MDIs). The Clean Air Act requires FDA, in consultation with the EPA, to determine whether an FDA-regulated product that releases an ODS is an essential use of the ODS. FDA has concluded that there are no substantial technical barriers to formulating flunisolide, triamcinolone, metaproterenol, pirbuterol, albuterol and ipratropium in combination, cromolyn, and nedocromil as products that do not release ODSs, and therefore they will no longer be essential uses of ODSs as of the effective dates of this rule. MDIs for these active moieties containing an ODS may not be marketed after the relevant effective date. DATES: Removal of Part 2.125(e)(2)(iii) and 2.125(e)(4)(vii) is effective June 14, 2010. Removal of Part 2.125(e)(1)(v) and 2.125(e)(4)(iv) is effective December 31, 2010. Removal of Part 2.125(e)(1)(iii) is effective June 30, 2011. Removal of 2.125(e)(2)(iv) and Part 2.125(e)(4)(viii) is effective December 31, 2013. /Ozone-Depleting Substances/
75 FR 19213 (4/14/2010). Available from, as of March 14, 2013: https://www.gpo.gov/fdsys/browse/collection.action?collectionCode=FR
Use of ozone-depleting substances in foods, drugs, devices, or cosmetics. (a) As used in this section, ozone-depleting substance (ODS) means any class I substance as defined in 40 CFR part 82, appendix A to subpart A, or class II substance as defined in 40 CFR part 82, appendix B to subpart A. (b) Except as provided in paragraph (c) of this section, any food, drug, device, or cosmetic that is, consists in part of, or is contained in an aerosol product or other pressurized dispenser that releases an ODS is not an essential use of the ODS under the Clean Air Act. (c) A food, drug, device, or cosmetic that is, consists in part of, or is contained in an aerosol product or other pressurized dispenser that releases an ODS is an essential use of the ODS under the Clean Air Act if paragraph (e) of this section specifies the use of that product as essential. For drugs, including biologics and animal drugs, and for devices, an investigational application or an approved marketing application must be in effect, as applicable. ... (e) The use of ODSs in the following products is essential: ... (2) Metered-dose short-acting adrenergic bronchodilator human drugs for oral inhalation. Oral pressurized metered-dose inhalers containing the following active moieties: ... (iv) Pirbuterol. ... (4) Other essential uses. (iii) Anesthetic drugs for topical use on accessible mucous membranes of humans where a cannula is used for application. ... (vi) Metered-dose atropine sulfate aerosol human drugs administered by oral inhalation. ... (viii) Metered-dose ipratropium bromide and albuterol sulfate, in combination, administered by oral inhalation for human use. (ix) Sterile aerosol talc administered intrapleurally by thoracoscopy for human use. /Ozone-Depleting Substances/
21 CFR 2.125 (USFDA); U.S. National Archives and Records Administration's Electronic Code of Federal Regulations. Available from, as of March 14, 2013: https://www.ecfr.gov/cgi-bin/ECFR?page=browse
All fluorocarbons will undergo thermal decomposition when exposed to flame or red-hot metal. Decomposition products of the chlorofluorocarbons will include hydrofluoric & hydrochloric acid along with smaller amounts of phosgene & carbonyl fluoride. The last compound is very unstable to hydrolysis & quickly changes to hydrofluoric acid & carbon dioxide in the presence of moisture.
International Labour Office. Encyclopaedia of Occupational Health and Safety. 4th edition, Volumes 1-4 1998. Geneva, Switzerland: International Labour Office, 1998., p. 104.185
In contact with open flame or very hot surface fluorocarbons may decomp into highly irritant & toxic gases: chlorine, hydrogen fluoride or chloride, & even phosgene.
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-159
Achievements in Stratospheric Ozone Protection Progress Report: This report covers the important and substantial achievements of the people, programs, and organizations that are working to protect the Earth's ozone layer. As impressive as these accomplishments are, our work is not done. Even though we have reduced or eliminated the use of many ozone-depleting substances, some still remain. Additionally, since ozone-depleting substances persist in the air for long periods of time, the past use of these substances continues to affect the ozone layer today. We must also continue to ensure that the alternatives being brought to the market support the country's long-term environmental goals in a cost-effective manner.[EPA; Achievements in Stratospheric Ozone Protection Progress Report, Available from, as of March 11, 2013: http://www.epa.gov/ozone/downloads/spd-annual-report_final.pdf]
UNEP; Ozone Secretariat. Twenty Questions and Answers about the Ozone Layer: 2010 Update. The questions address the nature of atmospheric ozone, the chemicals that cause ozone depletion, how global and polar ozone depletion occur, the success of the Montreal Protocol, and what could lie ahead for the ozone layer.[Available from, as of May 21, 2013: http://ozone.unep.org/Assessment_Panels/SAP/Scientific_Assessment_2010/]
USEPA; Ozone Layer Protection - Alternatives/SNAP Program. List of Substitutes. Substitutes are reviewed on the basis of ozone depletion potential, global warming potential, toxicity, flammability, and exposure potential as described in the final SNAP rule (59 FR 13044). Lists of acceptable and unacceptable substitutes are updated several times each year.[Available from, as of May 21, 2013: www.epa.gov/ozone/snap/lists/index.html]
Neurotoxin - Acute solvent syndrome
... If inhalation occurs, epinephrine or other sympathomimetic amines & adrenergic activators should not be admin since they will further sensitize heart to development of arrhythmias.
Clayton, G. D. and F. E. Clayton (eds.). Patty's Industrial Hygiene and Toxicology: Volume 2A, 2B, 2C: Toxicology. 3rd ed. New York: John Wiley Sons, 1981-1982., p. 3101
Victims of freon inhalation require management for hypoxic, CNS anesthetic, & cardiac symptoms. Patients must be removed from the exposure environment, & high flow supplemental oxygen should be utilized. The respiratory system should be evaluated for injury, aspiration, or pulmonary edema & treated appropriately. CNS findings should be treated supportively. A calm environment with no physical exertion is imperative to avoid increasing endogenous adrenegic levels. Exogenous adrenergic drugs must not be used to avoid inducing sensitized myocardial dysrhythmias. Atropine is ineffective in treating bradyarrhythmias. For ventricular dysrhythmias, diphenylhydantoin & countershock may be effective. Cryogenic dermal injuries should be treated by water bath rewarming at 40-42 °C until vasodilatory flush has returned. Elevation of the limb & standard frostbite management with late surgical debridement should be utilized. Ocular exposure requires irrigation & slit lamp evaluation for injury. /Freons/
Haddad, L.M., Clinical Management of Poisoning and Drug Overdose. 2nd ed. Philadelphia, PA: W.B. Saunders Co., 1990., p. 1282
Immediate first aid: Ensure that adequate decontamination has been carried out. If patient is not breathing, start artificial respiration, preferably with a demand-valve resuscitator, bag-valve-mask device, or pocket mask, as trained. Perform CPR as necessary. Immediately flush contaminated eyes with gently flowing water. Do not induce vomiting. If vomiting occurs, lean patient forward or place on left side (head-down position, if possible) to maintain an open airway and prevent aspiration. Keep patient quiet and maintain normal body temperature. Obtain medical attention. /Chlorinated fluorocarbons (CFCs) and related compounds/
Currance, P.L. Clements, B., Bronstein, A.C. (Eds).; Emergency Care For Hazardous Materials Exposure. 3Rd edition, Elsevier Mosby, St. Louis, MO 2005, p. 221
Basic treatment: Establish a patent airway (oropharyngeal or nasopharyngeal airway, if needed). Suction if necessary. Watch for signs of respiratory insufficiency and assist ventilations as needed. Administer oxygen by nonrebreather mask at 10 to 15 L/min. Minimize physical activity and provide a quiet atmosphere. Monitor for pulmonary edema and treat if necessary ... . Anticipate seizures and treat if necessary ... . For eye contamination, flush eyes immediately with water. Irrigate each eye continuously with 0.9% saline (NS) during transport ... . Do not use emetics. Rinse mouth and administer 5 mL/kg up to 200 mL of water for dilution if the patient can swallow, has a strong gag reflex, and does not drool. Administer activated charcoal ... . Treat frostbite with rapid rewarming techniques ... . /Chlorinated fluorocarbons (CFCs) and related compounds/
Currance, P.L. Clements, B., Bronstein, A.C. (Eds).; Emergency Care For Hazardous Materials Exposure. 3Rd edition, Elsevier Mosby, St. Louis, MO 2005, p. 222
Advanced treatment: Consider orotracheal or nasotracheal intubation for airway control in the patient who is unconscious, has severe pulmonary edema, or is in severe respiratory distress. Positive-pressure ventilation techniques with a bag-valve-mask device may be beneficial. Consider drug therapy for pulmonary edema ... . Monitor cardiac rhythm and treat arrhythmias if necessary ... . Start IV administration of D5W /SRP: "To keep open", minimal flow rate/. Use 0.9% saline (NS) or lactated Ringer's (LR) if signs of hypovolemia are present. For hypotension with signs of hypovolemia,administer fluid cautiously. Watch for signs of fluid overload ... . Treat seizures with diazepam or lorazepam ... . Use proparacaine hydrochloride to assist eye irrigation ... . /Chlorinated fluorocarbons (CFCs) and related compounds/
Currance, P.L. Clements, B., Bronstein, A.C. (Eds).; Emergency Care For Hazardous Materials Exposure. 3Rd edition, Elsevier Mosby, St. Louis, MO 2005, p. 222
For more Antidote and Emergency Treatment (Complete) data for 1,1-DICHLORO-1,2,2,2-TETRAFLUOROETHANE (7 total), please visit the HSDB record page.
Initial Medical Screening: Employees should be screened for history of certain medical conditions which might place the employee at increased risk from Refrigerant 114 exposure. /These are/ chronic respiratory and cardiovascular disease. Periodic Medical Examination: Any employee developing /these/ conditions should be referred for further medical examination. /Freon 114/
Mackison, F. W., R. S. Stricoff, and L. J. Partridge, Jr. (eds.). NIOSH/OSHA - Occupational Health Guidelines for Chemical Hazards. DHHS(NIOSH) Publication No. 81-123 (3 VOLS). Washington, DC: U.S. Government Printing Office, Jan. 1981., p. 1
/SIGNS AND SYMPTOMS/ Early ... human experience indicated that high vapor concn (eg, 20%) may cause confusion, pulmonary irritation, tremors, & rarely coma, but ... these effects were generally transient & without late sequelae.
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-159
/SIGNS AND SYMPTOMS/ Aerosol sprays containing fluorocarbon propellants are another source of solvent intoxication. Prolonged exposure or daily use may result in damage to several organ systems. Clinical problems include cardiac arrhythmias, bone marrow depression, cerebral degeneration, and damage to liver, kidney, & peripheral nerves. Death occasionally has been attributed to inhalant abuse, probably via the mechanism of cardiac arrhythmias, especially accompanying exercise or upper airway obstruction. /Fluorocarbon propellants/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 575
/SIGNS AND SYMPTOMS/ Obviously the cause of death is in considerable doubt. Laryngeal spasm or edema, oxygen displacement, or sensitization of the myocardium to endogenous catecholamines with subsequent ventricular fibrillation appear to be reasonable possibilities.
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-159
/SURVEILLANCE/ In a cross-sectional study the neurological effects of fluorocarbons were evaluated in 27 refrigeration repair workers. Fourteen age matched reference subjects were selected from a local union of plumbers, pipe-fitters, and insulation workers. A case of peripheral neuropathy in a commercial refrigeration repairman prompted the investigation. Personal air samples from 2 worker-participants over the course of a typical workshift showed 1.4 ppm chlorodifluoromethane and 2.2 ppm chloropentafluorethane. There were no cases of peripheral neuropathy in the study subjects. There was no significant difference in mean nerve conduction velocities (ulnar, median, peroneal, sural, tibial) between study and reference subjects. Lightheadedness and palpitations were reported significantly more often by refrigeration repair workers (p < 0.05).
Campell DD et al; Br J Ind Med 43: 107-11 (1986)
For more Human Toxicity Excerpts (Complete) data for 1,1-DICHLORO-1,2,2,2-TETRAFLUOROETHANE (6 total), please visit the HSDB record page.
/LABORATORY ANIMALS: Acute Exposure/ Early animal ... experience indicated that high vapor concn (eg, 20%) may cause confusion, pulmonary irritation, tremors, & rarely coma, but that these effects were generally transient & without late sequelae.
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-159
/LABORATORY ANIMALS: Acute Exposure/ In experimental animals variable degrees of tachycardia, myocardial depression, and hypotension have been described.
Hamilton, A., and H. L. Hardy. Industrial Toxicology. 3rd ed. Acton, Mass.: Publishing Sciences Group, Inc., 1974., p. 293
/LABORATORY ANIMALS: Acute Exposure/ ... Chlorofluorocarbons could sensitize the canine myocardium to adrenaline, resulting in serious cardiac arrhythmias. /CFCs/
Rom, W.N. (ed.). Environmental and Occupational Medicine. 2nd ed. Boston, MA: Little, Brown and Company, 1992., p. 1300
/LABORATORY ANIMALS: Acute Exposure/ Long term exposure of rats and rabbits to atmospheres containing 1 percent Dichlorotetrafluoroethane, two hours a day, five days a week, induced no sign of intoxication in these animals. Considering the individual variations of the amount of red and white blood cells observed in the rat, no significant variation has been observed in this animal during the length of the experimentation. Histological examination shows no trace of lesion which could be attributed to the toxicity of the product. The discrete hepatic lesions observed in one of the treated rabbits have also been found in the control animals, and the same holds true for the lesions of acute pneumopathy, of broncho-pneumonial type, found in two rats who died during the period of experimentation. No significant change of the electroencephalographic tracings were observed in the rabbit. (French)
Desoille H et al; Archives des Maladies Professionnelles de Medecine du Travail et de Securite Sociale, Vol. 34, No. 3, p. 117-125 (1973)
It is possible that patients with cardiac or resp disorders may prove especially susceptible to /aerosol propellants/. /Propellants/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 910
1,1-Dichloro-1,2,2,2-tetrafluoroethane's former production and possible use as an aerosol propellant, refrigerant, solvent, fire extinguisher, blowing agent, or dielectric fluid may have resulted in its release to the environment through various waste streams. Fully halogenated chlorofluorocarbons (CFCs), such as 1,1-dichloro-1,2,2,2-tetrafluoroethane, were scheduled for production phase-out in 1987 by the Montreal Protocol. Although originally scheduled for 50% production phase-out by the year 2000 in developed countries, the worsening ozone depletion has forced acceleration of the CFC phase-out. If released to air, a vapor pressure of 1640 mm Hg at 25 °C indicates 1,1-dichloro-1,2,2,2-tetrafluoroethane will exist solely as a gas in the ambient atmosphere. 1,1-Dichloro-1,2,2,2-tetrafluoroethane does not contain any groups that react with photochemically produced hydroxyl radicals or atmospheric ozone. In addition 1,1-dichloro-1,2,2,2-tetrafluoroethane does not contain any chromophores that absorb solar radiation >290 nm and, therefore, it is not susceptible to direct photolysis in the troposphere. Therefore, 1,1-dichloro-1,2,2,2-tetrafluoroethane will be extremely persistent in the troposphere. In the stratosphere, 1,1-dichloro-1,2,2,2-tetrafluoroethane will slowly photolyze, releasing chlorine atoms which in turn are responsible for removing ozone. Calculated stratospheric lifetimes for other completely halogenated fluorochloroethanes are generally hundreds of years. If released to soil, 1,1-dichloro-1,2,2,2-tetrafluoroethane is expected to have moderate mobility based upon an estimated Koc of 200. Volatilization from moist soil surfaces is expected to be an important fate process based upon an estimated Henry's Law constant of 1.2 atm-cu m/mole. 1,1-Dichloro-1,2,2,2-tetrafluoroethane may volatilize from dry soil surfaces based upon its vapor pressure. Biodegradation data in soil or water were not available. If released into water, 1,1-dichloro-1,2,2,2-tetrafluoroethane is expected to moderately adsorb to suspended solids and sediment based upon the estimated Koc. Volatilization from water surfaces is expected to be an important fate process based upon this compound's estimated Henry's Law constant. Estimated volatilization half-lives for a model river and model lake are 3.8 hours and 5.2 days, respectively. An estimated BCF of 32 suggests the potential for bioconcentration in aquatic organisms is moderate. Occupational exposure to 1,1-dichloro-1,2,2,2-tetrafluoroethane may occur through inhalation and dermal contact with this compound at workplaces where it is produced or used. Limited monitoring data indicate that the general population may be exposed to 1,1-dichloro-1,2,2,2-tetrafluoroethane via inhalation of ambient air. (SRC)
1,1-Dichloro-1,2,2,2-tetrafluoroethane's former production and use as an aerosol propellant, refrigerant, solvent, fire extinguisher, blowing agent, or dielectric fluid(1) may have resulted in its release to the environment through various waste streams(SRC). Fully halogenated chlorofluorocarbons (CFCs), such as 1,1-dichloro-1,2,2,2-tetrafluoroethane, were scheduled for production phaseout in 1989 by the Montreal Protocol(2). Although originally scheduled for 50% production phaseout by the year 1998 in developed countries, the worsening ozone depletion has forced acceleration of the CFC phase-out to total phaseout by the year 2000(1).
(1) Rusch GM; Patty's Toxicology. (1999-2012). New York, NY: John Wiley & Sons, Organic Chlorofluoro Hydrocarbons. On-line posting date: Aug 17, 2012
(2) Smart BE, Fernandez RE; Kirk-Othmer Encyclopedia of Chemical Technology. (1999-2012). New York, NY: John Wiley & Sons; Fluorinated Aliphatic Compounds. Online Posting Date: December 4, 2000
TERRESTRIAL FATE: Based on a classification scheme(1), an estimated Koc value of 200 (SRC), determined from a structure estimation method(2), indicates that 1,1-dichloro-1,2,2,2-tetrafluoroethane is expected to have moderate mobility in soil(SRC). Volatilization of 1,1-dichloro-1,2,2,2-tetrafluoroethane from moist soil surfaces is expected to be an important fate process(SRC) given an estimated Henry's Law constant of 1.2 atm-cu m/mole, derived from its vapor pressure, 1640 mm Hg(4), and water solubility, 137 mg/L(5). The potential for volatilization of 1,1-dichloro-1,2,2,2-tetrafluoroethane from dry soil surfaces may exist, based upon a vapor pressure of 1640 mm Hg(4). Biodegradation data in soil were not available(SRC, 2012).
(1) Swann RL et al; Res Rev 85: 17-28 (1983)
(2) Meylan WM et al; Environ Sci Technol 26: 1560-67 (1992)
(3) Meylan WM, Howard PH; Environ Toxicol Chem 10: 1283-93 (1991)
(4) Daubert TE, Danner RP; Physical and Thermodynamic Properties of Pure Chemicals Data Compilation. Washington, DC: Taylor and Francis (1995)
(5) Hine J, Mookerjee PK; J Org Chem 40: 292-8 (1975)
AQUATIC FATE: Based on a classification scheme(1), an estimated Koc value of 200(SRC), determined from an estimation method(2), indicates that 1,1-dichloro-1,2,2,2-tetrafluoroethane may moderately adsorb to suspended solids and sediment(SRC). Volatilization from water surfaces is expected(3) based upon an estimated Henry's Law constant of 1.2 atm-cu m/mole, derived from its vapor pressure, 1640 mm Hg(4), and water solubility, 137 mg/L(8). Using this Henry's Law constant and an estimation method(3), volatilization half-lives for a model river and model lake are 3.8 hours and 5.2 days, respectively(SRC). According to a classification scheme(5), an estimated BCF of 32(SRC), from an estimated log Kow of 2.78(6) and a regression-derived equation(7), suggests the potential for bioconcentration in aquatic organisms is moderate. Biodegradation data in water were not available(SRC, 2012).
(1) Swann RL et al; Res Rev 85: 17-28 (1983)
(2) Meylan WM et al; Environ Sci Technol 26: 1560-67 (1992)
(3) Lyman WJ et al; Handbook of Chemical Property Estimation Methods. Washington, DC: Amer Chem Soc pp. 4-9, 15-1 to 15-29 (1990)
(4) Daubert TE, Danner RP; Physical and Thermodynamic Properties of Pure Chemicals Data Compilation. Washington, DC: Taylor and Francis (1995)
(5) Franke C et al; Chemosphere 29: 1501-14 (1994)
(6) US EPA; Estimation Program Interface (EPI) Suite. Ver. 4.1. Jan, 2011. Available from, as of Dec 14, 2012: https://www.epa.gov/oppt/exposure/pubs/episuitedl.htm
(7) Meylan WM et al; Environ Toxicol Chem 18: 664-72 (1999)
(8) Hine J, Mookerjee PK; J Org Chem 40: 292-8 (1975)
ATMOSPHERIC FATE: According to a model of gas/particle partitioning of semivolatile organic compounds in the atmosphere(1), 1,1-dichloro-1,2,2,2-tetrafluoroethane, which has a vapor pressure of 1640 mm Hg at 25 °C(2), is expected to exist solely as a gas if released to the ambient atmosphere. 1,1-Dichloro-1,2,2,2-tetrafluoroethane is not expected to degrade in the troposphere and it will disperse and slowly diffuse to the stratosphere, a process that may take decades(3). In the stratosphere, 1,1-dichloro-1,2,2,2-tetrafluoroethane will slowly photolyze, releasing chlorine atoms which in turn are responsible for removing ozone(4). While some 1,1-dichloro-1,2,2,2-tetrafluoroethane may be lost from the atmosphere by being scavenged by rain, any loss will be returned to the atmosphere by volatilization(SRC). 1,1-Dichloro-1,2,2,2-tetrafluoroethane does not contain any chromophores that absorb solar radiation >290 nm and, therefore, it is not susceptible to direct photolysis in the troposphere(SRC).
(1) Bidleman TF; Environ Sci Technol 22: 361-367 (1988)
(2) Daubert TE, Danner RP; Physical and Thermodynamic Properties of Pure Chemicals Data Compilation. Washington, DC: Taylor and Francis (1995)
(3) Dilling WL; pp. 154-97 in Environmental Risk Analysis for Chemicals Conway RA ed New York, NY: Van Nostrand Reinhold (1982)
(4) Chou CC et al; J Phys Chem 82: 1-7 (1978)
Hydroxyl radicals and ozone are the two most important atmospheric species affecting atmospheric persistence. 1,1-Dichloro-1,2,2,2-tetrafluoroethane does not contain any groups that react with photochemically produced hydroxyl radicals. Similarly, it also does not contain any groups that would react with atmospheric ozone. In addition 1,1-dichloro-1,2,2,2-tetrafluoroethane does not contain any chromophores that absorb solar radiation >290 nm and therefore make it susceptible to direct photolysis in the troposphere. Therefore, 1,1-dichloro-1,2,2,2-tetrafluoroethane will be extremely persistent in the troposphere. In the stratosphere, 1,1-dichloro-1,2,2,2-tetrafluoroethane will slowly photolyze, releasing chlorine atoms which in turn are responsible for removing ozone(1). Reaction with singlet oxygen should be an additional stratospheric sink but no data on reaction rates are available(2). Calculated stratospheric lifetimes for other completely halogenated fluorochloroethanes are generally hundreds of years(1). While no experimental data on hydrolysis of 1,1-dichloro-1,2,2,2-tetrafluoroethane could be found, the rate of hydrolysis of Freon (chlorofluorocarbon) compounds is extremely low(2).
(1) Chou CC et al; J Phys Chem 82: 1-7 (1978)
(2) Du Pont de Nemours Co; Freon Products Information B-2; A-98825 12/80 (1980)
An estimated BCF of 32 was calculated for 1,1-dichloro-1,2,2,2-tetrafluoroethane(SRC), using an estimated log Kow of 2.78(1) and a regression-derived equation(2). According to a classification scheme(3), this BCF suggests the potential for bioconcentration in aquatic organisms is moderate.
(1) US EPA; Estimation Program Interface (EPI) Suite. Ver. 4.1. Jan, 2011. Available from, as of Dec 14, 2012: https://www.epa.gov/oppt/exposure/pubs/episuitedl.htm
(2) Meylan WM et al; Environ Toxicol Chem 18: 664-72 (1999)
(3) Franke C et al; Chemosphere 29: 1501-14 (1994)
Using a structure estimation method based on molecular connectivity indices(1), the Koc for 1,1-dichloro-1,2,2,2-tetrafluoroethane can be estimated to be 200(SRC). According to a classification scheme(2), this estimated Koc value suggests that 1,1-dichloro-1,2,2,2-tetrafluoroethane is expected to have moderate mobility in soil.
(1) Meylan WM et al; Environ Sci Technol 26: 1560-67 (1992)
(2) Swann RL et al; Res Rev 85: 17-28 (1983)
The Henry's Law constant for 1,1-dichloro-1,2,2,2-tetrafluoroethane is estimated as 1.2 atm-cu m/mole, derived from its vapor pressure, 1640 mm Hg(1), and water solubility, 137 mg/L(2). This Henry's Law constant indicates that 1,1-dichloro-1,2,2,2-tetrafluoroethane is expected to volatilize rapidly from water surfaces(3). Based on this Henry's Law constant, the volatilization half-life from a model river (1 m deep, flowing 1 m/sec, wind velocity of 3 m/sec)(3) is estimated as 3.8 hours(SRC). The volatilization half-life from a model lake (1 m deep, flowing 0.05 m/sec, wind velocity of 0.5 m/sec)(3) is estimated as 5.2 days(SRC). 1,1-Dichloro-1,2,2,2-tetrafluoroethane's estimated Henry's Law constant(1,2) indicates that volatilization from moist soil surfaces may occur(SRC). The potential for volatilization of 1,1-dichloro-1,2,2,2-tetrafluoroethane from dry soil surfaces may exist based upon its vapor pressure(1).
(1) Daubert TE, Danner RP; Physical and Thermodynamic Properties of Pure Chemicals Data Compilation. Washington, DC: Taylor and Francis (1995)
(2) Hine J, Mookerjee PK; J Org Chem 40: 292-8 (1975)
(3) Lyman WJ et al; Handbook of Chemical Property Estimation Methods. Washington, DC: Amer Chem Soc pp. 15-1 to 15-29 (1990)
1,1-Dichloro-1,2,2,2-tetrafluoroethane may be distributed globally in the air, in both the troposphere and stratosphere, by analogy to CFC 114 a chlorofluorocarbon with an atmospheric lifetime of 200 years, found in the air in both the troposphere and stratosphere(1).
(1) Rusch GM; Patty's Toxicology. (1999-2012). New York, NY: John Wiley & Sons, Organic Chlorofluoro Hydrocarbons. On-line posting date: Aug 17, 2012
According to the 2006 TSCA Inventory Update Reporting data, the number of persons reasonably likely to be exposed in the industrial manufacturing, processing, and use of 1,1-dichloro-1,2,2,2-tetrafluoroethane is 100 to 999; the data may be greatly underestimated(1).
(1) US EPA; Inventory Update Reporting (IUR). Non-confidential 2006 IUR Records by Chemical, including Manufacturing, Processing and Use Information. Washington, DC: U.S. Environmental Protection Agency. Available from, as of Dec 19, 2012: https://cfpub.epa.gov/iursearch/index.cfm
Occupational exposure to 1,1-dichloro-1,2,2,2-tetrafluoroethane may occur through inhalation and dermal contact with this compound at workplaces where it is produced or used. Limited monitoring data indicate that the general population may be exposed to 1,1-dichloro-1,2,2,2-tetrafluoroethane via inhalation of ambient air. (SRC)
Associated Occupational Diseases with Exposure to the Compound
Solvents, acute toxic effect [Category: Acute Poisoning]
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=BAMUEXIPKSRTBS-UHFFFAOYSA-N
- Australian Industrial Chemicals Introduction Scheme (AICIS)Ethane, 1,1-dichloro-1,2,2,2-tetrafluoro-https://services.industrialchemicals.gov.au/search-inventory/
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/1,1-Dichloro-1,2,2,2-tetrafluoroethanehttps://commonchemistry.cas.org/detail?cas_rn=374-07-2
- ChemIDplus1,1-Dichloro-1,2,2,2-tetrafluoroethanehttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000374072ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA Chemical Data Reporting (CDR)LICENSEThe U.S. Government retains a nonexclusive, royalty-free license to publish or reproduce these documents, or allow others to do so, for U.S. Government purposes. These documents may be freely distributed and used for non-commercial, scientific and educational purposes.https://www.epa.gov/web-policies-and-procedures/epa-disclaimers#copyrightEthane, 1,1-dichloro-1,2,2,2-tetrafluoro-https://www.epa.gov/chemical-data-reporting
- EPA Chemicals under the TSCAEthane, 1,1-dichloro-1,2,2,2-tetrafluoro-https://www.epa.gov/chemicals-under-tscaEPA TSCA Classificationhttps://www.epa.gov/tsca-inventory
- EPA DSSTox1,1-Dichlorotetrafluoroethanehttps://comptox.epa.gov/dashboard/DTXSID9027150CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-notice1,1-dichloro-1,2,2,2-tetrafluoroethanehttps://echa.europa.eu/substance-information/-/substanceinfo/100.006.159
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking1,1-DICHLORO-1,2,2,2-TETRAFLUOROETHANEhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/8AWA8IET6R
- Hazardous Substances Data Bank (HSDB)1,1-DICHLORO-1,2,2,2-TETRAFLUOROETHANEhttps://pubchem.ncbi.nlm.nih.gov/source/hsdb/5564
- Haz-Map, Information on Hazardous Chemicals and Occupational DiseasesLICENSECopyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://haz-map.com/About1,1-Dichloro-1,2,2,2-tetrafluoroethanehttps://haz-map.com/Agents/2703
- Japan Chemical Substance Dictionary (Nikkaji)
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawEthane, 1,1-dichloro-1,2,2,2-tetrafluoro-http://www.nist.gov/srd/nist1a.cfm
- SpectraBaseEthane, 1,1-dichloro-1,2,2,2-tetrafluoro-https://spectrabase.com/spectrum/LD1VCph8vMOEthane, 1,1-dichloro-1,2,2,2-tetrafluoro-https://spectrabase.com/spectrum/Fen4oGy64pW2,2-DICHLORO-1,1,1,2-TETRAFLUORO-ETHANEhttps://spectrabase.com/spectrum/CBjV1MkEwFn1,1-DICHLOROTETRAFLUOROETHANE (F114A)https://spectrabase.com/spectrum/6r2iylQWjF2BAMUEXIPKSRTBS-UHFFFAOYSA-Nhttps://spectrabase.com/spectrum/4axj0KJrpJg
- NMRShiftDB
- Springer Nature
- SpringerMaterials1,1-dichloro-1,2,2,2-tetrafluoroethanehttps://materials.springer.com/substanceprofile/docs/smsid_xakzwgxpnajzvqds
- Wikidata1,1-dichloro-1,2,2,2-tetrafluoroethanehttps://www.wikidata.org/wiki/Q161277
- WikipediaKrypton hexafluoridehttps://en.wikipedia.org/wiki/Krypton_hexafluoride1,1-Dichlorotetrafluoroethanehttps://en.wikipedia.org/wiki/1,1-Dichlorotetrafluoroethane
- PubChemPFAS and Fluorinated Compounds in PubChemhttps://gitlab.com/uniluxembourg/lcsb/eci/pubchem-docs/-/raw/main/pfas-tree/PFAS_Tree.pdf?inline=false
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- CAMEO ChemicalsLICENSECAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data.https://cameochemicals.noaa.gov/help/reference/terms_and_conditions.htm?d_f=falseCAMEO Chemical Reactivity Classificationhttps://cameochemicals.noaa.gov/browse/react
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403032887https://pubchem.ncbi.nlm.nih.gov/substance/403032887
- NCBI
CONTENTS

