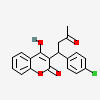
Coumachlor
- COUMACHLOR
- 81-82-3
- Tomorin
- Cumachlor
- Ratilan
- Create:2011-12-26
- Modify:2024-12-28
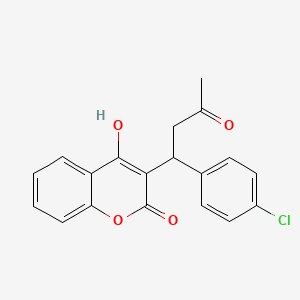
- 3-(1-(4-chlorophenyl)-3-oxobutyl)-4-hydroxy-2H-1-benzopyran-2-one
- 3-(alpha-acetonyl-p-chlorobenzyl)-4-hydroxycoumarin
- coumachlor
- COUMACHLOR
- 81-82-3
- Tomorin
- Cumachlor
- Ratilan
- p-Chlorowarfarin
- Coumachlore
- Cumachloor
- Famarin
- Kumachlor
- Experimental rodenticide 332
- Geigy rodenticide exp. 332
- 3-(1-(4-Chlorophenyl)-3-oxobutyl)-4-hydroxy-2H-chromen-2-one
- 3-(1-Acetyl-2-(p-chlorophenyl)ethyl)-4-hydroxycoumarin
- 3-[1-(4-chlorophenyl)-3-oxobutyl]-4-hydroxychromen-2-one
- UCD8XZW42P
- G-23133
- 3-(1-(4-Chlorophenyl)-3-oxobutyl)-4-hydroxy-2H-1-benzopyran-2-one
- 2H-1-Benzopyran-2-one, 3-[1-(4-chlorophenyl)-3-oxobutyl]-4-hydroxy-
- 3-(1-(4-Chlorophenyl)-3-oxobutyl)-4-hydroxycoumarin
- DTXSID8041797
- CHEBI:80741
- 4-Hydroxy-3-(1-(4-chlorophenyl)-3-oxobutyl)-2H-1-benzopyran-2-one
- 3-(1-(4-Chloorfenyl)-3-oxo-butyl)-4-hydroxy-cumarine
- 3-(1-(4-Chlor-phenyl)-3-oxo-butyl)-4-hydroxy-cumarin
- 3-(1-(4-Cloro-fenil)-3-oxo-butil)-4-idrossicumarina
- 3-(1-(4-Chlorophenyl)-3-oxo-butyl)-4-hydroxy-coumarine
- 2H-1-Benzopyran-2-one, 3-(1-(4-chlorophenyl)-3-oxobutyl)-4-hydroxy-
- Kumachlor [Czech]
- Cumachloor [Dutch]
- Cumachlor [German]
- 3-(.alpha.-Acetonyl-p-chlorobenzyl)-4-hydroxycoumarin
- Coumachlore [French]
- 3-(.alpha.-Acetonyl-4-chlorobenzyl)-4-hydroxycoumarin
- Coumarin, 3-(.alpha.-acetonyl-p-chlorobenzyl)-4-hydroxy-
- dl-3-(.alpha.-Acetonyl-4-chlorobenzyl)-4-hydroxycoumarin
- Caswell No. 004A
- 3-(.alpha.-p-Chlorophenyl-.beta.-acetylethyl)-4-hydroxycoumarin
- Coumachlor [ISO:BSI]
- Coumachlore [ISO-French]
- (+/-)-Coumachlor
- HSDB 7116
- EINECS 201-378-1
- UNII-UCD8XZW42P
- EPA Pesticide Chemical Code 224200
- 3-(alpha-Acetonyl-p-chlorobenzyl)-4-hydroxycoumarin
- BRN 1689759
- Coumachlor, 98%
- 3-(alpha-Acetonyl-4-chlorobenzyl)-4-hydroxycoumarin
- 3-(alpha-p-Chlorophenyl-beta-acetylethyl)-4-hydroxycoumarin
- COUMACHLOR [MI]
- 3-(1-(4-Chloorfenyl)-3-oxo-butyl)-4-hydroxy-cumarine [Dutch]
- COUMACHLOR [ISO]
- 3-(1-(4-Chlor-phenyl)-3-oxo-butyl)-4-hydroxy-cumarin [German]
- 3-(1-(4-Chlorophenyl)-3-oxo-butyl)-4-hydroxy-coumarine [French]
- 3-(1-(4-Cloro-fenil)-3-oxo-butil)-4-idrossicumarina [Italian]
- (.+/-.)-Coumachlor
- 3-(alpha-(p-Chlorphenyl)-beta-acetylaethyl)-4-hydroxycumarin [German]
- 5-18-04-00163 (Beilstein Handbook Reference)
- SCHEMBL433455
- 3-((alpha-Acetonyl)-4-chlorbenzyl)-4-hydroxycumarin
- SCHEMBL1477912
- CHEMBL1272169
- Coumarin, 3-(alpha-acetonyl-p-chlorobenzyl)-4-hydroxy-
- DTXCID6021797
- COUMACHLOR, (+/-)-
- 3-((alpha-Acetonyl)-4-chlorbenzyl)-4-hydroxycumarin [IUPAC]
- 3-(alpha-(p-Chlorphenyl)-beta-acetylaethyl)-4-hydroxycumarin
- Tox21_301860
- MFCD00012094
- P-CHLOROWARFARIN, (+/-)-
- ZINC03875546
- AKOS015962273
- CS-W013916
- CAS-81-82-3
- NCGC00255455-01
- AC-22308
- LS-14700
- NS00000510
- Coumachlor, PESTANAL(R), analytical standard
- H10531
- Q5176191
- 4-Hydroxy-3-[3-oxo-1-(4-chlorophenyl)butyl]coumarin
- (+/-)-3-(alpha-Acetonyl-p-chlorobenzyl)-4-hydroxycoumarin
- 3-(.alpha.-(p-Chlorphenyl)-.beta.-acetylaethyl)-4-hydroxycumarin
- 3-(1-(P-CHLOROPHENYL)-2-ACETYLETHYL)-4-HYDROXYCOUMARIN
- 3-[1-(4-Chlorophenyl)-3-oxobutyl]-4-hydroxy-2H-chromen-2-one #
- 3-((.ALPHA.-ACETONYL)-4-CHLORBENZYL)-4-HYDROXYCUMARIN [HSDB]
- 3-(.ALPHA.-ACETONYL-4-CHLOROBENZYL)-4-HYDROXY COUMARIN, (+/-)-
163.039 999
343.0733 862
285.0313 240
181.0414 98
325.063 7
163.039 999
285.0313 862
181.0414 127
189.0546 18
343.0733 7

H373 (100%): May causes damage to organs through prolonged or repeated exposure [Warning Specific target organ toxicity, repeated exposure]
H412 (100%): Harmful to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard]
P260, P273, P319, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 38 reports by companies from 1 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
STOT RE 2 (100%)
Aquatic Chronic 3 (100%)
Specific target organ toxicity (repeated exposure) - category 2
Hazardous to the aquatic environment (chronic) - category 3
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=DEKWZWCFHUABHE-UHFFFAOYSA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/(±)-Coumachlorhttps://commonchemistry.cas.org/detail?cas_rn=81-82-3
- ChemIDplusCoumachlor [ISO:BSI]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000081823ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSTox(+/-)-Coumachlorhttps://comptox.epa.gov/dashboard/DTXSID8041797CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeCoumachlor (EC: 201-378-1)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/44424
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Hazardous Substances Data Bank (HSDB)
- ChEBI
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.html
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jsp
- EU Pesticides Database
- Haz-Map, Information on Hazardous Chemicals and Occupational DiseasesLICENSECopyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://haz-map.com/AboutCoumachlorhttps://haz-map.com/Agents/4524
- Hazardous Chemical Information System (HCIS), Safe Work Australia
- NITE-CMCcoumachlor (ISO); 3-[1-(4-chlorophenyl)-3-oxobutyl]-4-hydroxycoumarin - FY2008 (New/original classication)https://www.chem-info.nite.go.jp/chem/english/ghs/08-mhlw-0011e.html
- Regulation (EC) No 1272/2008 of the European Parliament and of the CouncilLICENSEThe copyright for the editorial content of this source, the summaries of EU legislation and the consolidated texts, which is owned by the EU, is licensed under the Creative Commons Attribution 4.0 International licence.https://eur-lex.europa.eu/content/legal-notice/legal-notice.htmlcoumachlor (ISO); 3-[1-(4-chlorophenyl)-3-oxobutyl]-4-hydroxycoumarinhttps://eur-lex.europa.eu/eli/reg/2008/1272/oj
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citing
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.html
- MassBank Europe
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawCoumachlorhttp://www.nist.gov/srd/nist1a.cfm
- SpectraBase
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- Springer Nature
- Wikidatacoumachlorhttps://www.wikidata.org/wiki/Q5176191
- WikipediaCoumachlorhttps://en.wikipedia.org/wiki/Coumachlor
- Wiley
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlAnticoagulantshttps://www.ncbi.nlm.nih.gov/mesh/68000925Rodenticideshttps://www.ncbi.nlm.nih.gov/mesh/68012378
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403434956https://pubchem.ncbi.nlm.nih.gov/substance/403434956