Paclitaxel
- paclitaxel
- TAXOL
- 33069-62-4
- P88XT4IS4D
- Taxol A
- Create:2005-03-26
- Modify:2025-02-01
 Paclitaxel Ceribate (is active moiety of); Paclitaxel Poliglumex (is active moiety of);
Paclitaxel Ceribate (is active moiety of); Paclitaxel Poliglumex (is active moiety of);  7-Acetyltaxol (annotation moved to) ... View More ...
7-Acetyltaxol (annotation moved to) ... View More ...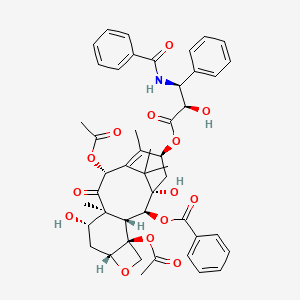
- 7 epi Taxol
- 7-epi-Taxol
- Anzatax
- Bris Taxol
- NSC 125973
- NSC-125973
- NSC125973
- Onxol
- Paclitaxel
- Paclitaxel, (4 alpha)-Isomer
- Paxene
- Praxel
- Taxol
- Taxol A
- Taxol, Bris
- paclitaxel
- TAXOL
- 33069-62-4
- P88XT4IS4D
- Taxol A
- Yewtaxan
- Genaxol
- Plaxicel
- Abraxane
- Ebetaxel
- Genetaxyl
- Capxol
- Paxene
- Onxol
- Cyclopax
- Genexol
- Intaxel
- Mitotax
- TaxAlbin
- OncoGel
- Pacliex
- Paxceed
- EmPAC
- Onxal
- Zisu
- Taxus stent
- Taxus Liberte
- ABI-007
- Padexol
- EndoTAG 1
- LipoPac
- Tocosol Paclitaxel
- (-)-Paclitaxel
- Nanoxel
- Paclitaxol
- Sindaxel
- NSC-125973
- Coroflex Please
- Cypher select
- Taxus Express
- LEP-ETU
- Genexol-PM
- (NAB)-Paclitaxel
- MBT 0206
- Infinnium
- Taxus
- HSDB 6839
- ABI 007
- DHP 107
- DHP-107
- Abraxane I.V. Suspension
- BMS 181339-01
- BMS-181339-01
- UNII-P88XT4IS4D
- DRG-0190
- Paclitaxel (Taxol)
- MFCD00869953
- NK 105
- NSC125973
- Paclitaxel (taxus canadensis)
- QW 8184
- CCRIS 8143
- Liposome-entrapped paclitaxel easy-to-use
- DTXSID9023413
- CHEBI:45863
- ABI-007 COMPONENT PACLITAXEL
- IG 001
- NK-105
- 5beta,20-Epoxy-1,2-alpha,4,7beta,10beta,13alpha-hexahydroxytax-11-en-9-one 4,10-diacetate 2-benzoate 13-ester with (2R,3S)-N-benzoyl-3-phenylisoserine
- QW-8184
- CHEMBL428647
- DTXCID603413
- (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-9-(((2R,3S)-3-benzamido-2-hydroxy-3-phenylpropanoyl)oxy)-12-(benzoyloxy)-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxete-6,12b-diyl diacetate
- nab-paclitaxel
- ORAXOL COMPONENT PACLITAXEL
- Paclitaxel [USAN:USP:INN:BAN]
- Abraxane (albumin-bound suspension)
- ABRAXANE COMPONENT PACLITAXEL
- MBT-0206
- 7,11-Methano-1H-cyclodeca[3,4]benz[1,2-b]oxete, benzenepropanoic acid deriv.
- ABI 007 COMPONENT PACLITAXEL
- (2aR-(2aalpha,4beta,4abeta,6beta,9alpha(alpha R*,betaS*),11alpha,12alpha,12balpha))-beta-(Benzoylamino)-alpha-hydroxybenzenepropanoic acid 6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca(3,4)benz(1,2-b)oxet-9-yl ester
- NCGC00164367-01
- NAB-PACLITAXEL COMPONENT PACLITAXEL
- NSC 125973
- PACLITAXEL (MART.)
- PACLITAXEL [MART.]
- PACLITAXEL (USP-RS)
- PACLITAXEL [USP-RS]
- (1S,2S,3R,4S,7R,9S,10S,12R,15S)-4,12-bis(acetyloxy)-1,9-dihydroxy-15-{[(2R,3S)-2-hydroxy-3-phenyl-3-(phenylformamido)propanoyl]oxy}-10,14,17,17-tetramethyl-11-oxo-6-oxatetracyclo[11.3.1.0^{3,10}.0^{4,7}]heptadec-13-en-2-yl benzoate
- PACLITAXEL (EP MONOGRAPH)
- PACLITAXEL (USP IMPURITY)
- PACLITAXEL [EP MONOGRAPH]
- PACLITAXEL [USP IMPURITY]
- Anzatax
- Cynviloq
- PACLITAXEL (USP MONOGRAPH)
- PACLITAXEL [USP MONOGRAPH]
- Xorane
- Paclitaxel (USAN:USP:INN:BAN)
- Taxol (Paclitaxel)
- Bris Taxol
- Taxol, Bris
- [(1S,2S,3R,4S,7R,9S,10S,12R,15S)-4,12-diacetyloxy-15-[(2R,3S)-3-benzamido-2-hydroxy-3-phenylpropanoyl]oxy-1,9-dihydroxy-10,14,17,17-tetramethyl-11-oxo-6-oxatetracyclo[11.3.1.03,10.04,7]heptadec-13-en-2-yl] benzoate
- SMR000857385
- EndoTAG-1
- SR-01000075350
- paclitaxelum
- Nanotaxel
- Paclical
- Pacligel
- Paxoral
- Paclitaxel?
- Paclitaxel,(S)
- Abraxane (TN)
- (2alpha,5beta,7beta,10beta,13alpha)-4,10-bis(acetyloxy)-1,7-dihydroxy-13-({(2R,3S)-2-hydroxy-3-phenyl-3-[(phenylcarbonyl)amino]propanoyl}oxy)-9-oxo-5,20-epoxytax-11-en-2-yl benzoate
- [diacetoxy-[(2R,3S)-3-benzamido-2-hydroxy-3-phenyl-propanoyl]oxy-dihydroxy-tetramethyl-oxo-[?]yl] benzoate
- 4alpha,10beta-bis(acetyloxy)-13alpha-((2S,3S)-3-benzamido-2-hydroxy-3-phenylpropanoyloxy)-1,7beta-dihydroxy-9-oxo-5beta,20-epoxytax-11-en-2alpha-yl benzoate
- 4alpha,10beta-bis(acetyloxy)-13alpha-[(2S,3S)-3-benzamido-2-hydroxy-3-phenylpropanoyloxy]-1,7beta-dihydroxy-9-oxo-5beta,20-epoxytax-11-en-2alpha-yl benzoate
- Paclitaxel; 5beta,20-Epoxy-1,7beta-dihydroxy-9-oxotax-11-ene-2alpha,4,10beta,13alpha-tetrayl 4,10-diacetate 2-benzoate 13-[(2R,3S)-3-(benzoylamino)-2-hydroxy-3-phenylpropanoate]; Taxol; Docetaxel Anhydrous Impurity F; Docetaxel Impurity F
- CAS-33069-62-4
- BMS-181339
- Paclitaxel-SSMM-VIP
- Paclitaxel (Standard)
- P-SSMM-VIP
- PACLITAXEL [MI]
- PACLITAXEL [INN]
- PACLITAXEL [JAN]
- Prestwick3_000155
- PACLITAXEL [HSDB]
- PACLITAXEL [USAN]
- PACLITAXELPACLITAXEL
- TAXOL (TN)
- PACLITAXEL [VANDF]
- SCHEMBL3976
- 3PPC5TL76P
- Nova-12005
- PACLITAXEL [WHO-DD]
- Paclitaxel, Taxus brevifolia
- BIDD:PXR0046
- BSPBio_000290
- KBioGR_002509
- KBioSS_002517
- Paclitaxel (JAN/USP/INN)
- MLS002154218
- MLS002695976
- OAS-PAC-100
- PACLITAXEL [EMA EPAR]
- BPBio1_000320
- GTPL2770
- MEGxp0_001940
- Taxol (TN) (Bristol Meyers)
- PACLITAXEL [GREEN BOOK]
- PACLITAXEL [ORANGE BOOK]
- ACon1_002231
- HY-B0015R
- KBio2_002509
- KBio2_005077
- KBio2_007645
- KBio3_002987
- ANX-513
- DHP-208
- DTS-301
- L01CD01
- SDP-013
- cMAP_000068
- HMS2090D07
- HMS2095O12
- HMS2231A16
- HMS3712O12
- HY-B0015
- MPI-5018
- Tox21_112107
- BDBM50001839
- NSC745099
- AKOS007930675
- AKOS015969673
- AKOS025312303
- CCG-220155
- CS-1145
- DB01229
- GS-6554
- NSC-745099
- NCGC00164367-02
- NCGC00164367-03
- NCGC00164367-04
- NCGC00164367-05
- NCGC00164367-10
- Paclitaxel, From Taxus brevifolia, 95%
- (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-1,2a,3,4,4a,6,9,10,11,12,12a,12b-Dodecahydro-4,6,9,11,12,12b-hexahydroxy-4a,8,13,13-tetramethyl-7,11-methano-5H-cyclodeca(3,4)benz(1,2-b)oxet-5-one 6,12b-diacetate, 12-benzoate, 9-ester with (2R,3S)-N-benzoyl-3-phenylisoserine
- NCI60_000601
- Paclitaxel, from Taxus yannanensis, powder
- 1ST000431
- PACLITAXEL IMPURITY L [EP IMPURITY]
- AB00513812
- D00491
- EN300-117275
- M02242
- N88686
- AB00513812-02
- AB00513812-03
- Q423762
- 7,4]benz[1,2-b]oxete,benzenepropanoic acid deriv.
- SR-01000075350-1
- SR-01000075350-3
- SR-01000075350-6
- SR-01000075350-7
- SR-01000075350-9
- BRD-K62008436-001-03-1
- BRD-K62008436-001-05-6
- BRD-K62008436-001-22-1
- Paclitaxel, from semisynthetic (from Taxus sp.), >=97%
- Paclitaxel, European Pharmacopoeia (EP) Reference Standard
- Paclitaxel, from Taxus brevifolia, >=95% (HPLC), powder
- Paclitaxel, United States Pharmacopeia (USP) Reference Standard
- 12-benzoate, 9-ester with (2R,3S)-N-benzoyl-3-phenylisoserine
- Paclitaxel protein-bound particles for injectable suspension (albumin-bound)
- Paclitaxel, Pharmaceutical Secondary Standard; Certified Reference Material
- Paclitaxel natural for peak identification, European Pharmacopoeia (EP) Reference Standard
- (1S,2S,3R,4S,5R,7S,8S,10R,13S)-4,10-Diacetoxy-2-benzoyloxy-5,20-epoxy-1,7-dihydroxy-9-oxotax-11-en-13-yl (2R,3S)-3-benzoylamino-2-hydroxy-3-phenylpropionate
- (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-1,2a,3,4,4a,6,9,10,11,12,12a,12b-Dodecahydro 4,6,9,11,12,12b-hexahydroxy-4a,8,13,13-tetramethyl-7,11-methano 5Hcyclodeca(3,4)benz(1,2-b)oxet-5-one 6,12b-diacetate,
- (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-4,6,12b-Tris(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-11-hydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl (alphaR,betaS)-beta-(benzoylamino)-alpha-hydroxybenzenepropanoate
- (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl (aR,bS)-b-(benzoylamino)-a-hydroxybenzenepropanoate
- (2aR-(2aalpha,4beta,4abeta,6beta,9alpha(alpha R*,betaS*),11alpha,12alpha,12balpha))-beta-(Benzoylamino)-alpha-hydroxybenzenepropanoic acid 6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12
- (2beta,5beta,7alpha,8alpha,10alpha,13alpha)-4,10-bis(acetyloxy)-1,7-dihydroxy-13-({(2R,3S)-2-hydroxy-3-phenyl-3-[(phenylcarbonyl)amino]propanoyl}oxy)-9-oxo-5,20-epoxytax-11-en-2-yl benzoate
- ,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca(3,4)benz(1,2-b)oxet-9-yl ester
- ,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl ester, (alphaR,betaS)- (9CI)
- -cyclodeca[3,4]benz[1,2-b]oxet-9-yl ester, [2aR-[2aalpha,4beta,4abeta,6beta,9alpha(aR*,betaS*),11alpha,12alpha,12aalpha,12balpha]]-
- [(1S,2S,3R,4S,7R,9S,10S,12R,15S)-4,12-diacetyloxy-15-[(2R,3S)-3-benzamido-2-hydroxy-3-phenylpropanoyl]oxy-1,9-dihydroxy-10,14,17,17-tetramethyl-11-oxo-6-oxatetracyclo[11.3.1.03,10.04,7]heptadec-13-en-2-yl]benzoate
- 1203669-79-7
- 4,7beta,10beta-tris(acetyloxy)-13alpha-[[(2R,3S)-3-benzamido-2-hydroxy-3-phenylpropanoyl]oxy]-1-hydroxy-9-oxo-5beta,20-epoxytax-11-en-2alpha-yl benzoate
- 5-BETA,20-EPOXY-1,2-ALPHA,4,7-BETA,10-BETA,13-ALPHA-HEXAHYDROXY-TAX-11-EN-9-ONE 4,10-DIACETATE 2-BENZOATE 13-ESTER WITH (2R,3S)-N-BENZOYL-3-PHENYL-ISOSERINE
- 5beta,20-Epoxy-1,2 alpha, 4,7beta, 10beta, 13alpha-hexahydroxy tax-11-en-9-one 4,10-diacetate 2-benzoate 13-ester with (2R, 3S)-N-benzoyl-3-phenylisoserine
- BENZENEPROPANOIC ACID, .BETA.-(BENZOYLAMINO)-.ALPHA.-HYDROXY-, (2AR,4S,4AS,6R,9S,11S,12S,12AR,12BS)-6,12B-BIS(ACETYLOXY)-12-(BENZOYLOXY)-2A,3,4,4A,5,6,9,10,11,12,12A,12B-DODECAHYDRO-4,11-DIHYDROXY-4A,8,13,13-TETRAMETHYL-5-OXO-7,11-METHANO-1H-CYCLODECA(3,4)BENZ(1,2-B)OXET-9-YL ESTER, (.ALPHA.R,.BETA.S)-
- Benzenepropanoic acid, 6,12b-bis(acetyl oxy)-12-(benzoyloxy)- 2a,3,4,4a,5,6,9,10,11,12,12a,12b,- dodecahydro-4,11- dihydroxy-4a,8,13,13-tetramethyl-5-oxo- 7,11-methano- 1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl ester, [2aR- [2a.alpha.,4.beta.,4a.beta.,6.beta.,9.alpha.(alpha. R*,.beta.S*),11.alpha.,12.alpha.,12a.alpha.,12b.alpha.]]-
- Benzenepropanoic acid, b-(benzoylamino)-.alpha.-hydroxy-, (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl ester, (aR,bS)-
- Benzenepropanoic acid, beta-(benzoylamino)-alpha-hydroxy-, (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-4,6,12b-tris(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-11-hydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl ester, (alphaR,betaS)-
- Benzenepropanoic acid, beta-(benzoylamino)-alpha-hydroxy-, (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13
- Benzenepropanoic acid, beta-(benzoylamino)-alpha-hydroxy-, (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca(3,4)benz(1,2-b)oxet-9-yl ester, (alphaR,betaS)-
- Benzenepropanoic acid, beta-(benzoylamino)-alpha-hydroxy-, 4,6,12b-tris(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-11-hydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl ester, [2aR-[2aalpha,4beta,4abeta,6beta,9alpha(alphaR*,betaS*),11alpha,12alpha,12aalpha,12balpha]]-
- Benzenepropanoic acid, beta-(benzoylamino)-alpha-hydroxy-, 6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H
- Benzenepropanoic acid, beta-(benzoylamino)-alpha-hydroxy-, 6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca(3,4)benz(1,2-b)oxet-9-yl ester, (2aR-(2a-alpha,4-beta,4a-beta,6-beta,9-alpha(alpha-R*,beta-S*),11-alpha,12-alpha,12a-alpha, 12b-alpha))-
- Benzenepropanoic acid, beta-(benzoylamino)-alpha-hydroxy-, 6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca(3,4)benz(1,2-b)oxet-9-yl ester, (2aR-(2aalpha,4beta,4abeta,6beta,9alpha(alphaR*,betaS*),11alpha,12alpha,12aalpha,12balpha))-
- Paclitaxel semi-synthetic for peak identification, European Pharmacopoeia (EP) Reference Standard
- Paclitaxel semi-synthetic for system suitability, European Pharmacopoeia (EP) Reference Standard
- TAX-11-EN-9-ONE, 5-BETA,20-EPOXY-1,2-ALPHA,4,7-BETA,10-BETA,13-ALPHA-HEXA-HYDROXY-, 4,10-DIACETATE 2-BENZOATE 13-ESTER WITH (2R,3S)-N-BENZOYL-3-PHENYLISOSERINE
- Tax-11-en-9-one, 5beta,20-epoxy-1,2alpha,4,7beta,10beta,13alpha- hexahydroxy-, 4,10-diacetate 2-benzoate, 13-ester with (2R,3S)-N-benzoyl-3-phenylisoserine
- TAX-11-EN-9-ONE, 5BETA,20-EPOXY-1,2ALPHA,4,7BETA,10BETA,13ALPHA-HEXAHYDROXY-, 4,10-DIACETATE 2-BENZOATE 13-ESTER WITH (2R,3S)-N-BENZOYL-3-PHENYLISOSERINE
- Tax-11-en-9-one, 5beta,20-epoxy-1,2alpha,4,7beta,10beta,13alpha-hexahydroxy-, 4,10-diacetate 2-benzoate 13-ester with (2R,3S)-N-benzoyl-3-phenylisoserine (8CI)
- Tax-11-en-9-one, 5beta,20-epoxy-1,2alpha,4,7beta,10beta,13alpha-hexahydroxy-, 4,10-diacetate 2-benzoate, 13-ester with (2R,3S)-N-benzoyl-3-phenylisoserine
- Tax-11-en-9-one,20-epoxy-1,2.alpha.,4,7.beta., 10.beta.,13.alpha.- hexahydroxy-, 4,10-diacetate 2- benzoate,13-ester with (2R,3S)-N-benzoyl-3-phenylisoserine
105.03289 100
240.100906 46.45
122.059433 39.94
286.10614 21.33
263.141815 16.72
591.221118138149 0.07
308.0897076983055 0.07
531.1971982357466 0.05
411.1764266126977 0.04
533.2143411338807 0.03
509.21583238897574 0.09
286.1090091168227 0.06
165.06830009561898 0.04
309.14887707525037 0.04
551.2288473039301 0.04
Paclitaxel Ceribate (is active moiety of)
- Paclitaxel Poliglumex (is active moiety of)
7-Acetyltaxol (annotation moved to)
- Albumin-Bound Paclitaxel (annotation moved to)
Paclitaxel is approved to be used alone or with other drugs to treat:
• AIDS-relatedKaposi sarcoma. It is used as second-line therapy.
• Breast cancer. It is used:
• In patients with node-positive cancer. It is given as adjuvant therapy with doxorubicin hydrochloride-containing combination chemotherapy.
• In patients with metastatic cancer that did not respond to combination chemotherapy.
• Non-small cell lung cancer. It is used with cisplatin as first-line therapy in patients whose cancer cannot be treated with surgery or radiation therapy.
• Ovarian cancer that is advanced. It is used with cisplatin as first-line therapy or alone in patients who have already received other treatment.
Paclitaxel is also being studied in the treatment of other types of cancer.Paclitaxel is also available in a different form called paclitaxel albumin-stabilized nanoparticle formulation.
21.7 L/h/m2 [Dose 135 mg/m2, infusion duration 24 h]
23.8 L/h/m2 [Dose 175 mg/m2, infusion duration 24 h]
7 L/h/m2 [Dose 135 mg/m2, infusion duration 3 h]
12.2 L/h/m2 [Dose 175 mg/m2, infusion duration 3 h]
- Cytoplasm
- Extracellular
- Membrane
Use (kg; approx.) in Germany (2009): >25
Use (kg) in USA (2002): 39
Consumption (g per capita; approx.) in Germany (2009): 0.000305
Consumption (g per capita) in the USA (2002): 0.000139
Calculated removal (%): 62.3



H315 (87.5%): Causes skin irritation [Warning Skin corrosion/irritation]
H317 (74.2%): May cause an allergic skin reaction [Warning Sensitization, Skin]
H318 (85.9%): Causes serious eye damage [Danger Serious eye damage/eye irritation]
H334 (17.2%): May cause allergy or asthma symptoms or breathing difficulties if inhaled [Danger Sensitization, respiratory]
H335 (87.1%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation]
H340 (12.9%): May cause genetic defects [Danger Germ cell mutagenicity]
H341 (28.1%): Suspected of causing genetic defects [Warning Germ cell mutagenicity]
H360 (24.6%): May damage fertility or the unborn child [Danger Reproductive toxicity]
H361 (73.8%): Suspected of damaging fertility or the unborn child [Warning Reproductive toxicity]
H372 (14.1%): Causes damage to organs through prolonged or repeated exposure [Danger Specific target organ toxicity, repeated exposure]
H413 (57%): May cause long lasting harmful effects to aquatic life [Hazardous to the aquatic environment, long-term hazard]
P203, P233, P260, P261, P264, P264+P265, P270, P271, P272, P273, P280, P284, P302+P352, P304+P340, P305+P354+P338, P317, P318, P319, P321, P332+P317, P333+P317, P342+P316, P362+P364, P403, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 256 reports by companies from 27 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Skin Irrit. 2 (87.5%)
Skin Sens. 1 (74.2%)
Eye Dam. 1 (85.9%)
Resp. Sens. 1 (17.2%)
STOT SE 3 (87.1%)
Muta. 1B (12.9%)
Muta. 2 (28.1%)
Repr. 1B (24.6%)
Repr. 2 (73.8%)
STOT RE 1 (14.1%)
Aquatic Chronic 4 (57%)
Excerpt from ERG Guide 154 [Substances - Toxic and/or Corrosive (Non-Combustible)]:
TOXIC and/or CORROSIVE; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause environmental contamination. (ERG, 2024)
EYES: First check the victim for contact lenses and remove if present. Flush victim's eyes with water or normal saline solution for 20 to 30 minutes while simultaneously calling a hospital or poison control center. Do not put any ointments, oils, or medication in the victim's eyes without specific instructions from a physician. IMMEDIATELY transport the victim after flushing eyes to a hospital even if no symptoms (such as redness or irritation) develop.
SKIN: IMMEDIATELY flood affected skin with water while removing and isolating all contaminated clothing. Gently wash all affected skin areas thoroughly with soap and water. If symptoms such as redness or irritation develop, IMMEDIATELY call a physician and be prepared to transport the victim to a hospital for treatment.
INHALATION: IMMEDIATELY leave the contaminated area; take deep breaths of fresh air. If symptoms (such as wheezing, coughing, shortness of breath, or burning in the mouth, throat, or chest) develop, call a physician and be prepared to transport the victim to a hospital. Provide proper respiratory protection to rescuers entering an unknown atmosphere. Whenever possible, Self-Contained Breathing Apparatus (SCBA) should be used; if not available, use a level of protection greater than or equal to that advised under Protective Clothing.
INGESTION: DO NOT INDUCE VOMITING. If the victim is conscious and not convulsing, give 1 or 2 glasses of water to dilute the chemical and IMMEDIATELY call a hospital or poison control center. Be prepared to transport the victim to a hospital if advised by a physician. If the victim is convulsing or unconscious, do not give anything by mouth, ensure that the victim's airway is open and lay the victim on his/her side with the head lower than the body. DO NOT INDUCE VOMITING. IMMEDIATELY transport the victim to a hospital. (NTP, 1992)
Excerpt from ERG Guide 154 [Substances - Toxic and/or Corrosive (Non-Combustible)]:
IMMEDIATE PRECAUTIONARY MEASURE: Isolate spill or leak area in all directions for at least 50 meters (150 feet) for liquids and at least 25 meters (75 feet) for solids.
SPILL: Increase the immediate precautionary measure distance, in the downwind direction, as necessary.
FIRE: If tank, rail tank car or highway tank is involved in a fire, ISOLATE for 800 meters (1/2 mile) in all directions; also, consider initial evacuation for 800 meters (1/2 mile) in all directions. (ERG, 2024)
SMALL SPILLS AND LEAKAGE: If you spill this chemical, you should dampen the solid spill material with water, then transfer the dampened material to a suitable container. Use absorbent paper dampened with water to pick up any remaining material. Seal your contaminated clothing and the absorbent paper in a vapor-tight plastic bag for eventual disposal. Wash all contaminated surfaces with a soap and water solution. Do not reenter the contaminated area until the Safety Officer (or other responsible person) has verified that the area has been properly cleaned.
STORAGE PRECAUTIONS: You should protect this material from moisture, and store it in a freezer. (NTP, 1992)
Alcohols and Polyols
Amides and Imides
Esters, Sulfate Esters, Phosphate Esters, Thiophosphate Esters, and Borate Esters
Hydrocarbons, Aliphatic Unsaturated
Hazard Traits - Developmental Toxicity; Reproductive Toxicity
Authoritative List - Prop 65
Report - regardless of intended function of ingredient in the product
Paclitaxel has been associated with serum aminotransferase elevations in 7% to 26% of patients, but values greater than 5 times the upper limit of normal (ULN) in only 2% of those receiving the highest doses. Similar rates of alkaline phosphatase elevations and occasional mild bilirubin elevations also occur. The abnormalities are usually asymptomatic, mild and self-limited, rarely requiring dose modification or discontinuation. Paclitaxel has not been linked convincingly to instances of delayed, idiosyncratic clinically apparent liver injury with jaundice. However, the hypersensitivity reactions that occur with infusions of paclitaxel can be severe and accompanied by acute hepatic necrosis. The liver injury may be relatively mild and anicteric (Case 1), but can also be severe with rapid onset of multiorgan failure and death. At least one instance of acute liver failure following a hypersensitivity reaction to paclitaxel has been published in the literature and recent modifications of the product labels for paclitaxel and docetaxel mention the occurrence of toxic deaths following severe infusion reactions. Because paclitaxel is often given with other antineoplastic agents, liver injury arising during therapy cannot always be reliably attributed to paclitaxel rather than to other specific agents. Furthermore, paclitaxel in combination with other anticancer agents may be associated with reactivation of hepatitis B, increased risk of opportunistic viral infections, sinusoidal obstruction syndrome or sepsis, any of which can cause liver test abnormalities or clinically apparent liver injury.
Likelihood score: D (possible cause of acute hepatic necrosis associated with a hypersensitivity reaction to the initial infusions).
M Chen, V Vijay, Q Shi, Z Liu, H Fang, W Tong. FDA-Approved Drug Labeling for the Study of Drug-Induced Liver Injury, Drug Discovery Today, 16(15-16):697-703, 2011. PMID:21624500 DOI:10.1016/j.drudis.2011.05.007
M Chen, A Suzuki, S Thakkar, K Yu, C Hu, W Tong. DILIrank: the largest reference drug list ranked by the risk for developing drug-induced liver injury in humans. Drug Discov Today 2016, 21(4): 648-653. PMID:26948801 DOI:10.1016/j.drudis.2016.02.015
◉ Summary of Use during Lactation
Most sources consider breastfeeding to be contraindicated during maternal antineoplastic drug therapy. It might be possible to breastfeed safely during intermittent therapy with an appropriate period of breastfeeding abstinence. Some have suggested a breastfeeding abstinence period of 6 to 10 days, but more recent pharmacokinetic modeling using a worst-case scenario suggests that 6 days would be adequate to minimize both systemic and gut toxicity after the colostral phase.
Chemotherapy may adversely affect the normal microbiome and chemical makeup of breastmilk. Women who receive chemotherapy during pregnancy are more likely to have difficulty nursing their infant than typical mothers.
◉ Effects in Breastfed Infants
Relevant published information was not found as of the revision date.
◉ Effects on Lactation and Breastmilk
A telephone follow-up study was conducted on 74 women who received cancer chemotherapy at one center during the second or third trimester of pregnancy to determine if they were successful at breastfeeding postpartum. Only 34% of the women were able to exclusively breastfeed their infants, and 66% of the women reported experiencing breastfeeding difficulties. This was in comparison to a 91% breastfeeding success rate in 22 other mothers diagnosed during pregnancy, but not treated with chemotherapy. Other statistically significant correlations included: 1. mothers with breastfeeding difficulties had an average of 5.5 cycles of chemotherapy compared with 3.8 cycles among mothers who had no difficulties; and 2. mothers with breastfeeding difficulties received their first cycle of chemotherapy on average 3.4 weeks earlier in pregnancy. Of the 9 women who received a taxane-containing regimen, 7 had breastfeeding difficulties.
Occupational hepatotoxin - Secondary hepatotoxins: the potential for toxic effect in the occupational setting is based on cases of poisoning by human ingestion or animal experimentation.
Skin Sensitizer - An agent that can induce an allergic reaction in the skin.
Asthma - Reversible bronchoconstriction (narrowing of bronchioles) initiated by the inhalation of irritating or allergenic agents.
Asthma, occupational [Category: Airway Disease]
Contact dermatitis, allergic [Category: Skin Disease]
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=RCINICONZNJXQF-MZXODVADSA-N
- Avoid echinacea. Co-administration may decrease the effectiveness of immunosuppressants, and echinacea may induce CYP3A4 increasing paclitaxel metabolism.
- Exercise caution with grapefruit products. Grapefruit inhibits CYP3A4 metabolism, which may increase the serum concentration of paclitaxel.
- Exercise caution with St. John's Wort. This herb induces the CYP3A4 metabolism of paclitaxel and may reduce its serum concentration.
- BindingDBLICENSEAll data curated by BindingDB staff are provided under the Creative Commons Attribution 3.0 License (https://creativecommons.org/licenses/by/3.0/us/).https://www.bindingdb.org/rwd/bind/info.jsp
- Chemical Probes Portal
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jspAlbumin-Bound Paclitaxelhttps://ctdbase.org/detail.go?type=chem&acc=D000068196130-nm albumin-bound paclitaxelhttps://ctdbase.org/detail.go?type=chem&acc=C520255
- Drug Gene Interaction database (DGIdb)LICENSEThe data used in DGIdb is all open access and where possible made available as raw data dumps in the downloads section.http://www.dgidb.org/downloadsPACLITAXELhttps://www.dgidb.org/drugs/rxcui:56946
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_usePaclitaxelhttps://www.drugbank.ca/drugs/DB01229
- IUPHAR/BPS Guide to PHARMACOLOGYLICENSEThe Guide to PHARMACOLOGY database is licensed under the Open Data Commons Open Database License (ODbL) https://opendatacommons.org/licenses/odbl/. Its contents are licensed under a Creative Commons Attribution-ShareAlike 4.0 International License (http://creativecommons.org/licenses/by-sa/4.0/)https://www.guidetopharmacology.org/about.jsp#licenseGuide to Pharmacology Target Classificationhttps://www.guidetopharmacology.org/targets.jsp
- Therapeutic Target Database (TTD)
- Toxin and Toxin Target Database (T3DB)LICENSET3DB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (T3DB) and the original publication.http://www.t3db.ca/downloadsPaclitaxelhttp://www.t3db.ca/toxins/T3D4019
- California Office of Environmental Health Hazard Assessment (OEHHA)
- CAMEO ChemicalsLICENSECAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data.https://cameochemicals.noaa.gov/help/reference/terms_and_conditions.htm?d_f=falseCAMEO Chemical Reactivity Classificationhttps://cameochemicals.noaa.gov/browse/react
- ChEBI
- FDA Pharm ClassesLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingFDA Pharmacological Classificationhttps://www.fda.gov/ForIndustry/DataStandards/StructuredProductLabeling/ucm162549.htm
- LiverTox
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/Paclitaxelhttps://www.wikidata.org/wiki/Q423762LOTUS Treehttps://lotus.naturalproducts.net/
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licence
- California Safe Cosmetics Program (CSCP) Product Database
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusPaclitaxel [USAN:USP:INN:BAN]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0033069624ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DTP/NCILICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuse
- EPA DSSToxCompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-notice(2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-1,2a,3,4,4a,6,9,10,11,12,12a,12b-Dodecahydro-4,6,9,11,12,12b-hexahydroxy-4a,8,13,13-tetramethyl-7,11-methano-5H-cyclodeca(3,4)benz(1,2-b)oxet-5-one 6,12b-diacetate, 12-benzoate, 9-ester with (2R,3S)-N-benzoyl-3-phenylisoserinehttps://echa.europa.eu/substance-information/-/substanceinfo/100.127.725(2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-1,2a,3,4,4a,6,9,10,11,12,12a,12b-Dodecahydro-4,6,9,11,12,12b-hexahydroxy-4a,8,13,13-tetramethyl-7,11-methano-5H-cyclodeca(3,4)benz(1,2-b)oxet-5-one 6,12b-diacetate, 12-benzoate, 9-ester with (2R,3S)-N-benzoyl-3-phenylisoserine (EC: 608-826-9)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/106818
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking7-O-acetylpaclitaxelhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/3PPC5TL76P
- Hazardous Substances Data Bank (HSDB)
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingHMDB0015360_msms_451453https://hmdb.ca/metabolites/HMDB0015360#spectra
- Haz-Map, Information on Hazardous Chemicals and Occupational DiseasesLICENSECopyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://haz-map.com/AboutPaclitaxelhttps://haz-map.com/Agents/21415
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/PaclitaxelNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- DailyMed
- Drug Induced Liver Injury Rank (DILIrank) DatasetLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- European Medicines Agency (EMA)LICENSEInformation on the European Medicines Agency's (EMA) website is subject to a disclaimer and copyright and limited reproduction notices.https://www.ema.europa.eu/en/about-us/legal-noticeApealea (EMEA/H/C/004154)https://www.ema.europa.eu/en/medicines/human/EPAR/apealeaAbraxane (EMEA/H/C/000778)https://www.ema.europa.eu/en/medicines/human/EPAR/abraxanePazenir (EMEA/H/C/004441)https://www.ema.europa.eu/en/medicines/human/EPAR/pazenirPaxene (EMEA/H/C/000216)https://www.ema.europa.eu/en/medicines/human/EPAR/paxenepaclitaxel (P/0141/2021)https://www.ema.europa.eu/en/medicines/human/paediatric-investigation-plans/emea-002894-pip01-20
- Drugs and Lactation Database (LactMed)
- Drugs@FDALICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- WHO Model Lists of Essential MedicinesLICENSEPermission from WHO is not required for the use of WHO materials issued under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Intergovernmental Organization (CC BY-NC-SA 3.0 IGO) license.https://www.who.int/about/policies/publishing/copyright
- EU Clinical Trials Register
- FDA Orange BookLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- WHO Anatomical Therapeutic Chemical (ATC) ClassificationLICENSEUse of all or parts of the material requires reference to the WHO Collaborating Centre for Drug Statistics Methodology. Copying and distribution for commercial purposes is not allowed. Changing or manipulating the material is not allowed.https://www.whocc.no/copyright_disclaimer/
- National Drug Code (NDC) DirectoryLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- NCI Investigational Drugs
- Japan Chemical Substance Dictionary (Nikkaji)
- Japan Pharmaceuticals and Medical Devices Agency (PMDA)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlTherapeutic category of drugs in Japanhttp://www.genome.jp/kegg-bin/get_htext?br08301.kegUSP drug classificationhttp://www.genome.jp/kegg-bin/get_htext?br08302.kegAnatomical Therapeutic Chemical (ATC) classificationhttp://www.genome.jp/kegg-bin/get_htext?br08303.kegTarget-based classification of drugshttp://www.genome.jp/kegg-bin/get_htext?br08310.keg
- Natural Product Activity and Species Source (NPASS)
- MassBank Europe
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- Metabolomics Workbench
- Nature Chemical Biology
- Nature Chemistry
- NCI Cancer Drugs
- NIPH Clinical Trials Search of Japan
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.html
- PharmGKBLICENSEPharmGKB data are subject to the Creative Commons Attribution-ShareALike 4.0 license (https://creativecommons.org/licenses/by-sa/4.0/).https://www.pharmgkb.org/page/policies
- PharosLICENSEData accessed from Pharos and TCRD is publicly available from the primary sources listed above. Please respect their individual licenses regarding proper use and redistribution.https://pharos.nih.gov/about
- Protein Data Bank in Europe (PDBe)
- RCSB Protein Data Bank (RCSB PDB)LICENSEData files contained in the PDB archive (ftp://ftp.wwpdb.org) are free of all copyright restrictions and made fully and freely available for both non-commercial and commercial use. Users of the data should attribute the original authors of that structural data.https://www.rcsb.org/pages/policies
- Rhea - Annotated Reactions DatabaseLICENSERhea has chosen to apply the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/). This means that you are free to copy, distribute, display and make commercial use of the database in all legislations, provided you credit (cite) Rhea.https://www.rhea-db.org/help/license-disclaimer
- SpectraBasePACLITAXEL;TAXOL;REFERENCE-22https://spectrabase.com/spectrum/K9rtV9YNzic
- Springer Nature
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidatapaclitaxelhttps://www.wikidata.org/wiki/Q423762
- WikipediaPaclitaxelhttps://en.wikipedia.org/wiki/Paclitaxel
- Wiley
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlAntineoplastic Agents, Phytogenichttps://www.ncbi.nlm.nih.gov/mesh/68000972Tubulin Modulatorshttps://www.ncbi.nlm.nih.gov/mesh/68050257
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 388338683https://pubchem.ncbi.nlm.nih.gov/substance/388338683
- NCBI





