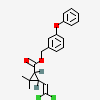
Permethrin
- Permethrin
- 52645-53-1
- Pounce
- Permethrine
- Permetrina
- Create:2005-03-27
- Modify:2025-01-18
- (m-Phenoxybenzyl)-cis,trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate
- 3-Phenoxybenzyl-(+-)-cis,trans-2,2-dichlorovinyl-2,2-dimethyl-cyclopropylcarboxylic acid, ester
- 3-Phenoxybenzyl-cis,trans-(1RS)-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate
- Ambush
- cis permethrin
- cis-(1RS)-permethrin
- cis-permethrin
- Cyclopropanecarboxylic Acid, 3-(2,2-dichloroethenyl)-2,2-dimethyl-, (3-phenoxyphenyl)methyl ester
- Elimite
- FMC 33297
- FMC-33297
- FMC33297
- NIA 33297
- NIA-33297
- NIA33297
- Nittifor
- NRDC 143
- NRDC 147
- NRDC-143
- NRDC-147
- NRDC143
- NRDC147
- Permethrin
- Permethrin, (1R-cis)-Isomer
- Permethrin, (1R-trans)-Isomer
- Permethrin, (1S-cis)-Isomer
- Permethrin, (1S-trans)-Isomer
- Permethrin, (cis)-Isomer
- Permethrin, (cis-(+-))-Isomer
- Permethrin, (trans)-Isomer
- Permethrin, (trans-(+-))-Isomer
- permethrin, cis-(1RS)-isomer
- Permethrin, trans-(1RS)-Isomer
- PP 557
- PP-557
- PP557
- S 3151
- S-3151
- S3151
- trans permethrin
- trans-(1RS)-Permethrin
- trans-permethrin
- Permethrin
- 52645-53-1
- Pounce
- Permethrine
- Permetrina
- Transpermethrin
- Permethrinum
- Acticin
- Corsair
- Dragnet
- Ectiban
- Imperator
- Kestrel
- Lyclear
- Outflank
- Perigen
- Permasect
- Perthrine
- Quamlin
- Stomoxi
- Stomoxin
- Coopex
- Cosair
- Dragon
- Eksmin
- Picket
- Pramex
- Exmin
- Expar
- Kafil
- Kavil
- Ridect pour-on
- Insorbcid MP
- Perigen W
- Permethrin,racemic
- Mitin BC
- Permanone 80
- 52341-32-9
- Elimite
- (3-phenoxyphenyl)methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropane-1-carboxylate
- Caswell No. 652BB
- Activyl tick plus
- 1RS,cis-Permethrin
- Permit
- UNII-509F88P9SZ
- CCRIS 2001
- ICI-PP 557
- SBP 15131TEC
- Transpermethrin [ISO]
- Permethrinum [Latin]
- DTXSID8022292
- CHEBI:34911
- HSDB 6790
- EINECS 258-067-9
- FMC 41655
- Permethrine [ISO-French]
- EPA Pesticide Chemical Code 109701
- NSC-760105
- JF 7065
- BRN 2063148
- Ambushfog
- Kaleait
- Stomozan
- WL 43479
- (+-)-cis-Permethrin
- Anomethrin N
- DTXCID102292
- 509F88P9SZ
- 1RS cis-Permethrin
- 3-phenoxybenzyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate
- 3-(2,2-Dichloroethenyl)-2,2-dimethylcyclopropane carboxylic acid, (3-phenoxyphenyl) methyl ester
- Cyclopropanecarboxylic acid, 3-(2,2-dichloroethenyl)-2,2-dimethyl-, (3-phenoxyphenyl)methyl ester
- Permethrinum (Latin)
- NSC 760105
- Cyclopropanecarboxylicacid, 3-(2,2-dichloroethenyl)-2,2-dimethyl-, (3-phenoxyphenyl)methyl ester,(1R,3S)-rel-
- NCGC00159390-02
- 1RS-trans-Permethrin
- Kudos
- trans-Permethrin D6 (dimethyl D6)
- Permethrine (ISO-French)
- 3-Phenoxybenzyl 2,2-dimethyl-3-(2,2-dichlorovinyl)cyclopropanecarboxylate
- trans-(+-)-Permethrin
- 3-Phenoxybenzyl (1RS)-cis-trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate
- 3-Phenoxybenzyl (1RS,3RS;1RS,3SR)-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate
- m-Phenoxybenzyl (+1)-cis,trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate
- (3-Phenoxyphenyl)methyl (+-)-cis,trans-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate
- 3-(Phenoxyphenyl)methyl (+-)-cis,trans-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate
- 93389-07-2
- Permethrin (ANSI:BSI:ISO)
- Permethrin [ANSI:BSI:ISO]
- NRDC-143
- S-3151
- Chinetrin
- Ecsumin
- Efmethrin
- Indothrin
- NRDC 146
- NRDC 148
- Exsmin
- Ipitox
- SBP-1513
- (+-)-trans-Permethrin
- Permethrine,c&t
- (+-)-cis-Fmc 33297
- Diffusil H
- Stomoxin P
- Outflank-stockade
- Dragnet FT
- Picket G
- [3-(phenyloxy)phenyl]methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate
- Permasect-25EC
- FMC 35171
- Cyclopropanecarboxylic acid, 3-(2,2-dichloroethenyl)-2,2-dimethyl-,(3-phenoxyphenyl)methyl ester, (1R,3R)-rel-
- Cyclopropanecarboxylic acid, 3-(2,2-dichlorovinyl)-2,2-dimethyl-, 3-phenoxybenzyl ester, (+-)-, (cis,trans)-
- SMR000778043
- Kestrel (pesticide)
- LE 79-519
- Antiborer 3768
- CAS-52645-53-1
- Bematin 987
- NRDC 143
- Permetrin (Hungarian)
- Permitrene (Hungarian)
- Permetrina [Portuguese]
- 82523-59-9
- FMC 33297
- NIA 33297
- PP 557
- Hemoglobin atlanta-coventry
- BRN 4153590
- Permethrn
- SBP-1513TEC
- Permethrin [USAN:INN:BAN]
- Lice Treatment
- AI3-29296
- MP79
- m-Phenoxybenzyl 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate
- BW-21-Z
- (m-Phenoxybenzyl)-cis,trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate
- S 3151
- Cyclopropanecarboxylic acid, 3-(2,2-dichlorovinyl)-2,2-dimethyl-, (3-phenoxyphenyl)methyl ester, (1R-trans)-
- OMS 1821
- Hb Atlanta-coventry
- Elimite (TN)
- MFCD00041809
- Hb At-Co
- Permethrin 5% w/w
- Leader Lice Treatment
- 52341-33-0
- Permethrin (Standard)
- Sunmark Lice Treatment
- Airline Pesticide Detox
- OUTFLANK STOCKADE
- AI3-29158
- Permethrin (USAN/INN)
- PERMETHRIN (IARC)
- Permethrin Cream 5% w/w
- cispermethrin (cis isomer)
- Health Mart Lice Treatment
- PERMETHRIN (MART.)
- Nix Complete Lice Treatment
- 3-(2,2-Dichloroethenyl)-2,2-dimethylcyclopropanecarboxylic acid (3-phenoxyphenyl)methyl ester
- biopermethrin (trans isomer)
- CHEMBL1525
- SCHEMBL26543
- Airline Pesticide Detox6030
- Cyclopropanecarboxylic acid, 3-(2,2-dichloroethenyl)-2,2-dimethyl-, (3-phenoxyphenyl)methyl ester, cis-(+-)-
- Cyclopropanecarboxylic acid, 3-(2,2-dichloroethenyl)-2,2-dimethyl-, (3-phenoxyphenyl)methyl ester, trans-(+-)-
- MLS001332525
- MLS001332526
- Nix Lice Killing Creme Rinse
- Permethrin cis/trans ~ 1:1
- Permethrin, analytical standard
- NDRC 143
- SCHEMBL15218274
- Topcare lice killing creme rinse
- HY-B0887R
- BW 21-Z
- P03AC04
- HMS2232L22
- HMS3264N07
- HMS3369D10
- Pharmakon1600-01504932
- Cyclopropanecarboxylic acid, 3-(2,2-dichlorovinyl)-2,2-dimethyl-, (3-phenoxyphenyl)methyl ester, (1R-cis)-
- good sense lice killing creme rinse
- HY-B0887
- SBP 1513
- Tox21_111627
- Tox21_201586
- Tox21_300691
- NSC760105
- s6461
- STL135986
- COMPONENT OF ACTIVYL TICK PLUS
- Good Neighbor Pharmacy Lice Treatment
- VECTRA 3D COMPONENT PERMETHRIN
- AKOS005746953
- CCG-213703
- DB04930
- KS-5079
- Permethrin 1000 microg/mL in Acetone
- (+-)-3-Phenoxybenzyl 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate
- (3-Phenoxyphenyl)methyl 3-(2,2-dichlorethenyl)-2,2-dimethylcyclopropanecarboxylate
- 3-Phenoxybenzyl(+-)-cis, trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane-1-carboxylate
- m-Phenoxybenzyl (+-)-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate
- PERMETHRIN (EMA EPAR VETERINARY)
- Permethrin 10 microg/mL in Cyclohexane
- USEPA/OPP Pesticide Code: 109701
- NCGC00159390-00
- NCGC00159390-04
- NCGC00159390-05
- NCGC00159390-06
- NCGC00159390-07
- NCGC00159390-08
- NCGC00159390-09
- NCGC00159390-10
- NCGC00159390-11
- NCGC00159390-12
- NCGC00159390-13
- NCGC00159390-14
- NCGC00254599-01
- NCGC00259135-01
- Permethrin 100 microg/mL in Cyclohexane
- PERMETHRIN COMPONENT OF VECTRA 3D
- 1ST20227
- 3-Phenoxybenzyl-(+-)-cis,trans-2,2-dichlorovinyl-2,2-dimethyl-cyclopropylcarboxylic acid, ester
- Permethrin (isomers), analytical standard
- DB-052153
- DB-214836
- Total Permethrin 100 microg/mL in Acetone
- ACTIVYL TICK PLUS COMPONENT PERMETHRIN
- NS00000502
- Permethrin, PESTANAL(R), analytical standard
- D05443
- G60893
- Nix Complete Maximum Strength Lice Elimination
- Nix FAMILY PACK Lice Killing Creme Rinse Kit
- SBI-0653467.0001
- AB00918441_05
- Permethrin is known as a pyrethroid insecticide.
- EN300-19628849
- MIXTURE OF CIS AND TRANS PERMETHRIN ISOMERS
- Q411635
- J-523915
- BRD-A25366096-001-07-3
- BRD-A25366096-001-08-1
- BRD-A25366096-001-09-9
- Permethrin (25:75), EuropePharmacopoeia (EP) Reference Standard
- (1RS,3SR)-3-(2,2-dichlorovinyl)-2,2-dimethylcyclo-propanecarboxylate
- 3-phenoxybenzyl 2-(2,2-dichlorovinyl)3,3-dimethylcyclopropanecarboxylate
- 3-Phenoxybenzyl 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate
- (3-phenoxyphenyl)methyl 3-(2,2-dichloroethenyl)-2,2-dimethyl-cyclopropane-1-carboxylate
- (3-Phenoxyphenyl)methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropane- carboxylate
- (3-phenoxyphenyl)methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate
- (3-phenoxyphenyl)methyl 3-(2,2-dichlorovinyl)-2,2-dimethyl-cyclopropanecarboxylate
- (3-phenoxyphenyl)methyl-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane- carboxylate
- 3-phenoxybenzyl (1RS)-cis,trans-3-(2,2-dichlorovinyl)- 2,2-dimethylcyclopropanecarboxylate
- 3-phenoxybenzyl (1RS)-cis,trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate
- 3-Phenoxybenzyl (1RS)-cis,trans-3-(2,2-dichlorvinyl)-2,2-dimethylcyclopropane- carboxylate
- m-phenoxybenzyl 2,2-dimethyl-3-(2',2'-dichlorovinyl)-cyclopropanecarboxylate
- m-Phenoxybenzyl(+1)-cis,trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane- carboxylate
- Permethrin for system suitability, EuropePharmacopoeia (EP) Reference Standard
- (3-Phenoxyphenyl)methyl (+-)cis,trans-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate
- (3-Phenoxyphenyl)methyl (+/-)-cis,trans-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate
- 3-(2,2-DICHLOROETHENYL)-2,2-DIMETHYLCYCLOPROPANE CARBOXYLIC ACID, (3-PHENOXY-PHENYL)METHYL ESTER
- 3-(2,2-Dichloroethenyl)-2,2-dimethylcyclopropanecarboxylic acid (3- phenoxyphenyl)methyl ester
149.0233 100
167.0338 46.13
71.0854 24.03
355.1093 5.54
183.0803 5.38
149.0234 100
71.0855 6.30
57.0699 4.43
121.0284 4.26
168.057 2.06
149.0233 999
167.0338 460
71.0854 240
355.1093 55
183.0803 53
149.0234 999
71.0855 119
167.0339 78
57.0699 43
183.0805 42
- Pediculicide (subclass of)
trans-Permethrin (annotation moved to)
- Extracellular
- Membrane
Use (kg; approx.) in Germany (2009): >250
Consumption (g per capita; approx.) in Germany (2009): 0.00305
Calculated removal (%): 97.4
Cosmetics product ingredient: Permethrin (including cis- and trans-)
Source: Permethrin is a synthetic chemical used as a pesticide and insect repellent. It is used in agriculture, veterinary health, and medication. Permethrin may be added to lotions intended to treat scabies (a skin infection caused by mites), and to sprays and shampoos used for lice control.
Potential health impacts: People may be exposed to permethrin by inhalation, ingestion, or through skin contact. Studies of scabies patients have found that dermal absorption of permethrin is low, but permethrin application may cause skin irritation. Inhaling permethrin may cause headache, nasal and respiratory irritation, dizziness, and nausea or vomiting. Studies of mice fed permethrin found increased rates of benign lung and liver tumors. The U.S. Environmental Protection Agency (U.S. EPA) classifies permethrin as likely to be carcinogenic to humans when ingested.
Product count: 2
Information on 256 consumer products that contain Permethrin in the following categories is provided:
• Commercial / Institutional
• Inside the Home
• Landscaping/Yard
• Personal Care
• Pesticides
• Pet Care


H302+H332 (11.8%): Harmful if swallowed or if inhaled [Warning Acute toxicity, oral; acute toxicity, inhalation]
H302 (99.8%): Harmful if swallowed [Warning Acute toxicity, oral]
H317 (88.9%): May cause an allergic skin reaction [Warning Sensitization, Skin]
H332 (99.8%): Harmful if inhaled [Warning Acute toxicity, inhalation]
H400 (99.6%): Very toxic to aquatic life [Warning Hazardous to the aquatic environment, acute hazard]
H410 (99.8%): Very toxic to aquatic life with long lasting effects [Warning Hazardous to the aquatic environment, long-term hazard]
P261, P264, P270, P271, P272, P273, P280, P301+P317, P302+P352, P304+P340, P317, P321, P330, P333+P317, P362+P364, P391, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 532 reports by companies from 19 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Reported as not meeting GHS hazard criteria per 1 of 532 reports by companies. For more detailed information, please visit ECHA C&L website.
There are 18 notifications provided by 531 of 532 reports by companies with hazard statement code(s).
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Acute Tox. 4 (99.8%)
Skin Sens. 1 (88.9%)
Acute Tox. 4 (99.8%)
Aquatic Acute 1 (99.6%)
Aquatic Chronic 1 (99.8%)
Acute toxicity (Oral) - Category 4
Specific target organ toxicity - Single exposure - Category 2 (nervous system)
Hazardous to the aquatic environment (Acute) - Category 1
Hazardous to the aquatic environment (Long-term) - Category 1
Excerpt from ERG Guide 171 [Substances (Low to Moderate Hazard)]:
Inhalation of material may be harmful. Contact may cause burns to skin and eyes. Inhalation of Asbestos dust may have a damaging effect on the lungs. Fire may produce irritating, corrosive and/or toxic gases. Some liquids produce vapors that may cause dizziness or asphyxiation. Runoff from fire control or dilution water may cause environmental contamination. (ERG, 2024)
Excerpt from ERG Guide 171 [Substances (Low to Moderate Hazard)]:
Some may burn but none ignite readily. Containers may explode when heated. Some may be transported hot. For UN3508, Capacitor, asymmetric, be aware of possible short circuiting as this product is transported in a charged state. Polymeric beads, expandable (UN2211) may evolve flammable vapours. (ERG, 2024)
Excerpt from ERG Guide 171 [Substances (Low to Moderate Hazard)]:
Refer to the "General First Aid" section. (ERG, 2024)
Excerpt from ERG Guide 171 [Substances (Low to Moderate Hazard)]:
CAUTION: Fire involving Safety devices (UN3268) and Fire suppressant dispersing devices (UN3559) may have a delayed activation and a risk of hazardous projectiles. Extinguish the fire at a safe distance.
SMALL FIRE: Dry chemical, CO2, water spray or regular foam.
LARGE FIRE: Water spray, fog or regular foam. Do not scatter spilled material with high-pressure water streams. If it can be done safely, move undamaged containers away from the area around the fire. Dike runoff from fire control for later disposal.
FIRE INVOLVING TANKS: Cool containers with flooding quantities of water until well after fire is out. Withdraw immediately in case of rising sound from venting safety devices or discoloration of tank. ALWAYS stay away from tanks in direct contact with flames. (ERG, 2024)
Excerpt from ERG Guide 171 [Substances (Low to Moderate Hazard)]:
IMMEDIATE PRECAUTIONARY MEASURE: Isolate spill or leak area in all directions for at least 50 meters (150 feet) for liquids and at least 25 meters (75 feet) for solids.
SPILL: Increase the immediate precautionary measure distance, in the downwind direction, as necessary.
FIRE: If tank, rail tank car or highway tank is involved in a fire, ISOLATE for 800 meters (1/2 mile) in all directions; also, consider initial evacuation for 800 meters (1/2 mile) in all directions. (ERG, 2024)
Excerpt from ERG Guide 171 [Substances (Low to Moderate Hazard)]:
Do not touch or walk through spilled material. Stop leak if you can do it without risk. Prevent dust cloud. For Asbestos, avoid inhalation of dust. Cover spill with plastic sheet or tarp to minimize spreading. Do not clean up or dispose of, except under supervision of a specialist.
SMALL DRY SPILL: With clean shovel, place material into clean, dry container and cover loosely; move containers from spill area.
SMALL SPILL: Pick up with sand or other non-combustible absorbent material and place into containers for later disposal.
LARGE SPILL: Dike far ahead of liquid spill for later disposal. Cover powder spill with plastic sheet or tarp to minimize spreading. Prevent entry into waterways, sewers, basements or confined areas. (ERG, 2024)
Excerpt from ERG Guide 171 [Substances (Low to Moderate Hazard)]:
Wear positive pressure self-contained breathing apparatus (SCBA). Structural firefighters' protective clothing provides thermal protection but only limited chemical protection. (ERG, 2024)
Esters, Sulfate Esters, Phosphate Esters, Thiophosphate Esters, and Borate Esters
Ethers
Halogenated Organic Compounds
Hydrocarbons, Aliphatic Unsaturated
◉ Summary of Use during Lactation
Because less than 2% is absorbed after topical application, rapid metabolism to inactive metabolites and safe application directly on infants' skin, topical permethrin products are acceptable in nursing mothers. Extensive exposure, such as from agricultural use or malaria control might have long-term health concerns because residues can be found in breastmilk. Only water-miscible cream, gel or liquid products should be applied to the breast because ointments may expose the infant to high levels of mineral paraffins via licking.
◉ Effects in Breastfed Infants
In a telephone follow-up study, 5 mothers who used permethrin during breastfeeding reported no adverse reactions in their breastfed infants.
◉ Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
Neurotoxin - Other CNS neurotoxin
Occupational hepatotoxin - Secondary hepatotoxins: the potential for toxic effect in the occupational setting is based on cases of poisoning by human ingestion or animal experimentation.
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=RLLPVAHGXHCWKJ-UHFFFAOYSA-N
- Australian Industrial Chemicals Introduction Scheme (AICIS)Cyclopropanecarboxylic acid, 3-(2,2-dichloroethenyl)-2,2-dimethyl-, (3-phenoxyphenyl)methyl esterhttps://services.industrialchemicals.gov.au/search-inventory/
- CAMEO ChemicalsLICENSECAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data.https://cameochemicals.noaa.gov/help/reference/terms_and_conditions.htm?d_f=falseCAMEO Chemical Reactivity Classificationhttps://cameochemicals.noaa.gov/browse/react
- ILO-WHO International Chemical Safety Cards (ICSCs)
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusTranspermethrin [ISO]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0052341329(+-)-cis-Permethrinhttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0052341330Permethrin [USAN:INN:BAN]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0052645531Hemoglobin atlanta-coventryhttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0093389072ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_usePermethrinhttps://www.drugbank.ca/drugs/DB04930
- DTP/NCILICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuse
- EPA DSSToxCompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- EPA Integrated Risk Information System (IRIS)
- EPA Safe Drinking Water Act (SDWA)
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticem-phenoxybenzyl 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylatehttps://echa.europa.eu/substance-information/-/substanceinfo/100.052.771m-phenoxybenzyl 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate (EC: 258-067-9)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/59336
- Hazardous Substances Data Bank (HSDB)
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingHMDB0015604_msms_443638https://hmdb.ca/metabolites/HMDB0015604#spectra
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/
- NJDOH RTK Hazardous Substance List
- Risk Assessment Information System (RAIS)LICENSEThis work has been sponsored by the U.S. Department of Energy (DOE), Office of Environmental Management, Oak Ridge Operations (ORO) Office through a joint collaboration between United Cleanup Oak Ridge LLC (UCOR), Oak Ridge National Laboratory (ORNL), and The University of Tennessee, Ecology and Evolutionary Biology, The Institute for Environmental Modeling (TIEM). All rights reserved.https://rais.ornl.gov/
- EU Pesticides Database
- California Safe Cosmetics Program (CSCP) Product DatabasePermethrin (including cis- and trans-)https://cscpsearch.cdph.ca.gov/search/detailresult/555
- Consumer Product Information Database (CPID)LICENSECopyright (c) 2024 DeLima Associates. All rights reserved. Unless otherwise indicated, all materials from CPID are copyrighted by DeLima Associates. No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://www.whatsinproducts.com/contents/view/1/6Consumer Products Category Classificationhttps://www.whatsinproducts.com/
- Haz-Map, Information on Hazardous Chemicals and Occupational DiseasesLICENSECopyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://haz-map.com/AboutPermethrinhttps://haz-map.com/Agents/6599
- ChEBI
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licence
- Toxin and Toxin Target Database (T3DB)LICENSET3DB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (T3DB) and the original publication.http://www.t3db.ca/downloadsPermethrinhttp://www.t3db.ca/toxins/T3D1850
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jsp
- Therapeutic Target Database (TTD)
- DailyMed
- European Medicines Agency (EMA)LICENSEInformation on the European Medicines Agency's (EMA) website is subject to a disclaimer and copyright and limited reproduction notices.https://www.ema.europa.eu/en/about-us/legal-noticeActivyl Tick Plus (EMEA/V/C/002234)https://www.ema.europa.eu/en/medicines/veterinary/EPAR/activyl-tick-plus
- Drugs and Lactation Database (LactMed)
- Drugs@FDALICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- WHO Model Lists of Essential MedicinesLICENSEPermission from WHO is not required for the use of WHO materials issued under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Intergovernmental Organization (CC BY-NC-SA 3.0 IGO) license.https://www.who.int/about/policies/publishing/copyright
- EPA Chemical and Products Database (CPDat)EPA CPDat Classificationhttps://www.epa.gov/chemical-research/chemical-and-products-database-cpdat
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/PERMETHRINNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- EPA Pesticide Ecotoxicity Database
- EPA Regional Screening Levels for Chemical Contaminants at Superfund Sites
- EU Clinical Trials Register
- USGS Health-Based Screening Levels for Evaluating Water-Quality DataLICENSEhttps://www.usgs.gov/legal
- NITE-CMC3-phenoxybenzyl 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate - FY2006 (New/original classication)https://www.chem-info.nite.go.jp/chem/english/ghs/06-imcg-0464e.html3-Phenoxybenzyl 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate (synonym: Permethrin) - FY2021 (Revised classification)https://www.chem-info.nite.go.jp/chem/english/ghs/21-meti-2023e.html
- Regulation (EC) No 1272/2008 of the European Parliament and of the CouncilLICENSEThe copyright for the editorial content of this source, the summaries of EU legislation and the consolidated texts, which is owned by the EU, is licensed under the Creative Commons Attribution 4.0 International licence.https://eur-lex.europa.eu/content/legal-notice/legal-notice.htmlpermethrin (ISO); m-phenoxybenzyl...https://eur-lex.europa.eu/eli/reg/2008/1272/oj
- FDA Orange BookLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Joint FAO/WHO Expert Committee on Food Additives (JECFA)LICENSEPermission from WHO is not required for the use of WHO materials issued under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Intergovernmental Organization (CC BY-NC-SA 3.0 IGO) licence.https://www.who.int/about/policies/publishing/copyright
- USGS Columbia Environmental Research CenterLICENSEhttps://www.usgs.gov/foia
- WHO Anatomical Therapeutic Chemical (ATC) ClassificationLICENSEUse of all or parts of the material requires reference to the WHO Collaborating Centre for Drug Statistics Methodology. Copying and distribution for commercial purposes is not allowed. Changing or manipulating the material is not allowed.https://www.whocc.no/copyright_disclaimer/
- International Agency for Research on Cancer (IARC)LICENSEMaterials made available by IARC/WHO enjoy copyright protection under the Berne Convention for the Protection of Literature and Artistic Works, under other international conventions, and under national laws on copyright and neighbouring rights. IARC exercises copyright over its Materials to make sure that they are used in accordance with the Agency's principles. All rights are reserved.https://publications.iarc.fr/Terms-Of-UseIARC Classificationhttps://www.iarc.fr/
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlEndocrine disrupting compoundshttp://www.genome.jp/kegg-bin/get_htext?br08006.kegUSP drug classificationhttp://www.genome.jp/kegg-bin/get_htext?br08302.kegAnatomical Therapeutic Chemical (ATC) classificationhttp://www.genome.jp/kegg-bin/get_htext?br08303.kegRisk category of Japanese OTC drugshttp://www.genome.jp/kegg-bin/get_htext?br08312.kegClassification of Japanese OTC drugshttp://www.genome.jp/kegg-bin/get_htext?br08313.kegAnimal drugs in Japanhttp://www.genome.jp/kegg-bin/get_htext?br08331.keg
- MassBank Europe
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- Metabolomics Workbench
- National Drug Code (NDC) DirectoryLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Natural Product Activity and Species Source (NPASS)
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.html
- PharmGKBLICENSEPharmGKB data are subject to the Creative Commons Attribution-ShareALike 4.0 license (https://creativecommons.org/licenses/by-sa/4.0/).https://www.pharmgkb.org/page/policies
- SpectraBasePERMETHRIN PESTANALhttps://spectrabase.com/spectrum/32FME7N6ZZptrans-3-(2,2-Dichloro-vinyl)-2,2-dimethyl-cyclopropanecarboxylic acid, 3-phenoxy-benzyl esterhttps://spectrabase.com/spectrum/17yCiV8hTs2cis-3-(2,2-Dichloro-vinyl)-2,2-dimethyl-cyclopropanecarboxylic acid, 3-phenoxy-benzyl esterhttps://spectrabase.com/spectrum/Er9P39Ws5LpPermethrin isomer-1https://spectrabase.com/spectrum/LHjUVYK9PbdPermethrin isomer-2https://spectrabase.com/spectrum/1piLjZOJAu
- Springer Nature
- Wikidatapermethrinhttps://www.wikidata.org/wiki/Q411635
- WikipediaDiethylhydroxylaminehttps://en.wikipedia.org/wiki/DiethylhydroxylaminePermethrinhttps://en.wikipedia.org/wiki/Permethrin
- Wiley
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlEnzyme Inhibitorshttps://www.ncbi.nlm.nih.gov/mesh/68004791Insecticideshttps://www.ncbi.nlm.nih.gov/mesh/68007306
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403406089https://pubchem.ncbi.nlm.nih.gov/substance/403406089
- NCBI