Liothyronine
- liothyronine
- triiodothyronine
- 3,3',5-Triiodo-L-thyronine
- 6893-02-3
- Liothyronin
- Create:2004-09-16
- Modify:2025-01-18
 Liothyronine Sodium (has salt form);
Liothyronine Sodium (has salt form);  Liotrix (is active moiety of); Levothyroxine; Liothyronine (component of) ... View More ...
Liotrix (is active moiety of); Levothyroxine; Liothyronine (component of) ... View More ...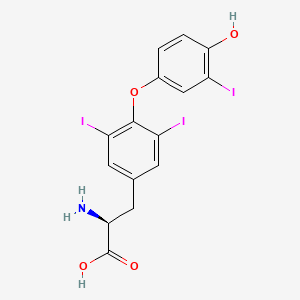
- 3,3',5-Triiodothyronine
- Cytomel
- Liothyronine
- Liothyronine Sodium
- T3 Thyroid Hormone
- Thyroid Hormone, T3
- Triiodothyronine
- liothyronine
- triiodothyronine
- 3,3',5-Triiodo-L-thyronine
- 6893-02-3
- Liothyronin
- Tresitope
- 3,5,3'-triiodothyronine
- Liotironina
- T3
- triothyrone
- O-(4-Hydroxy-3-iodophenyl)-3,5-diiodo-L-tyrosine
- Liothyroninum
- 3,5,3'-Triiodo-L-thyronine
- L-Liothyronine
- Lyothyronine
- Cyronine
- L-T3
- 3,3',5-Triiodothyronine
- 3,5,3'TRIIODOTHYRONINE
- L-3,3',5-TriioDOThyronine
- L-triiodothyronine
- (2S)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-diiodophenyl]propanoic acid
- Rathyronine, (s)-
- L-3,5,3'-Triiodothyronine
- CHEBI:18258
- (S)-2-amino-3-(4-(4-hydroxy-3-iodophenoxy)-3,5-diiodophenyl)propanoic acid
- Liotironina [INN-Spanish]
- Triiodo-L-thyronine
- L-Tyrosine, O-(4-hydroxy-3-iodophenyl)-3,5-diiodo-
- Liothyronine (GMP)
- Liothyronine (INN)
- 06LU7C9H1V
- DTXSID8023216
- 4-(4-Hydroxy-3-iodophenoxy)-3,5-diiodophenylalanine
- HSDB 3110
- EINECS 229-999-3
- MFCD00002593
- NSC 80203
- NSC-80203
- Thyronine, 3,3',5-triiodo-, L-
- Liothyroninum [INN-Latin]
- BRN 2710227
- CHEMBL1544
- UNII-06LU7C9H1V
- 4-(3-Iodo-4-hydroxyphenoxy)-3,5-diiodophenylalanine
- 4-(4-hydroxy-3-iodophenoxy)-3,5-diiodo-L-phenylalanine
- DTXCID403216
- 3,5,3'-triiodothyronine, l-
- Alanine, 3-(4-(4-hydroxy-3-iodophenoxy)-3,5-diiodophenyl)-, L-
- LIOTHYRONINE [INN]
- Liothyroninum (INN-Latin)
- Liotironina (INN-Spanish)
- T-3
- LIOTHYRONINE (USP-RS)
- LIOTHYRONINE [USP-RS]
- T3 (amino acid)
- LIOTHYRONINE (USP IMPURITY)
- LIOTHYRONINE [USP IMPURITY]
- Liothyronine [INN:BAN]
- (+)-Triiodothyronine
- CAS-6893-02-3
- T3 (VAN)
- Triiodothyronine, l-
- 3,5,3'-Tri-iodo-L-thyronine
- Triiodothyronine (T3)
- SR-05000000455
- NSC80203
- NSC-46046
- Therapeutic T3
- 3,3',5-Triiodothyronine, L-
- 4-(3-iodo-4-hydroxy-phenoxy)-3,5-diiodophenylalanine
- 1xzx
- (2S)-2-AMINO-3-(4-(4-HYDROXY-3-IODOPHENOXY)-3,5-DIIODOPHENYL)PROPANOIC ACID
- Prestwick_135
- Spectrum_001445
- L-3-(4-(4-Hydroxy-3-iodophenoxy)-3,5-diiodophenyl)alanine
- Triiodothyronine;3,3',5-Triiodo-L-thyronine;T3
- Liothyronine (Standard)
- Prestwick0_000853
- Prestwick1_000853
- Prestwick2_000853
- Prestwick3_000853
- Spectrum2_001984
- Spectrum3_001887
- Spectrum4_000326
- Spectrum5_001793
- LIOTHYRONINE [MI]
- Epitope ID:131324
- LIOTHYRONINE [HSDB]
- SCHEMBL8300
- NCIStruc1_000449
- LIOTHYRONINE [VANDF]
- REGID_for_CID_5920
- BSPBio_000865
- BSPBio_003394
- KBioGR_000671
- KBioGR_002568
- KBioSS_001925
- KBioSS_002577
- MLS000028458
- O-(4-Hydroxy-3-iodophenyl-3,5-diiodo-L-tyrosine
- 3,5,3'-triodo-L-thyronine
- LIOTHYRONINE [WHO-DD]
- SPBio_002167
- SPBio_002786
- BPBio1_000953
- GTPL2634
- HY-A0070AG
- HY-A0070AR
- L-3,3',5-triiodo-Thyronine
- BCBcMAP01_000192
- BDBM18860
- HY-A0070A
- KBio2_001925
- KBio2_002568
- KBio2_004493
- KBio2_005136
- KBio2_007061
- KBio2_007704
- KBio3_002897
- KBio3_003046
- H03AA02
- cMAP_000088
- HMS1570L07
- HMS2097L07
- HMS2235A22
- HMS3714L07
- TRIIODOTHYRONINE (T3 OR LIOTHYRONINE, ACTIVE) (6-11%)
- Tox21_110029
- Tox21_301943
- HB7470
- NCGC00013556
- s5726
- 3,5,3'-triiodo-L-thyronine (T3)
- (S)-2-Amino-3-(4-(4-hydroxy-3-iodophenoxy)-3,5-diiodophenyl)propanoicacid
- AKOS016003266
- 3,3',5-Triiodo-L-thyronine, 95%
- CCG-220853
- CS-4141
- DB00279
- L-3,3',5-Triiodothyronine, free acid
- SMP1_000179
- NCGC00013556-01
- NCGC00013556-02
- NCGC00013556-03
- NCGC00013556-04
- NCGC00013556-05
- NCGC00013556-12
- NCGC00013556-15
- NCGC00096669-01
- NCGC00096669-02
- NCGC00255336-01
- [125I]T3
- AC-31935
- AS-17446
- SMR000058402
- SBI-0206718.P001
- DB-021796
- CS-0622788
- NS00076841
- T0453
- A11632
- C02465
- D08128
- EN300-123970
- Q327362
- SR-01000003143
- SR-01000003143-4
- SR-05000000455-2
- SR-05000000455-3
- BRD-K89152108-001-03-3
- BRD-K89152108-236-05-0
- BRD-K89152108-236-06-8
- BRD-K89152108-236-07-6
- LEVOTHYROXINE SODIUM IMPURITY A [EP IMPURITY]
- 3,3',5-Triiodo-L-thyronine, >=95% (HPLC), powder
- Z1557400301
- D-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-diiodophenyl]alanine
- L-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-diiodophenyl]-Alanine
- Liothyronine, United States Pharmacopeia (USP) Reference Standard
- 3,3',5 Triiodothyronine (T3), IRMM(R) certified Reference Material
- O-(4-Hydroxy-3-iodophenyl)-3,5-diiodo-L-tyrosine,labeled with(125i)iodine
- Liothyronine, Pharmaceutical Secondary Standard; Certified Reference Material
- Tresitope , O-(4-Hydroxy-3-iodophenyl)-3,5-diiodo-L-tyrosine , Triiodothyronine , L-3,3',5-Triiodothyronine , 3,3',5-Triiodo-L-thyronine , Lyothyronine
204.77 Ų [M+H]+ [CCS Type: TW; Method: calibrated with polyalanine and drug standards]
191.01 Ų [M+Na]+ [CCS Type: TW; Method: calibrated with polyalanine and drug standards]
209.7 Ų [M+H]+ [CCS Type: DT; Method: single field calibrated with Agilent tune mix (Agilent)]
185.23 Ų [M+Na-2H]- [CCS Type: DT; Method: single field calibrated with Agilent tune mix (Agilent)]
209.1 Ų [M+H]+ [CCS Type: DT; Method: single field calibrated with ESI Low Concentration Tuning Mix (Agilent)]
194.3 Ų [M+Na]+ [CCS Type: DT; Method: single field calibrated with ESI Low Concentration Tuning Mix (Agilent)]
209.3 Ų [M+H]+
194.5 Ų [M+Na]+
126.905 100
72.0092 4.01
448.8542 2.17
605.7912 999
507.8656 185
478.8873 112
606.7929 86
651.7996 42
605.7907 999
651.7954 546
507.8644 134
606.7917 74
634.7694 61
225.078888 100
198.067276 73.04
605.788269 67.68
478.888397 45.26
507.863983 39.80
Liothyronine Sodium (has salt form)
Liotrix (is active moiety of)
- Levothyroxine; Liothyronine (component of)
- Levothyroxine; liothyronine; thyroid (component of)
- Adenosine triphosphate; ammonium carbonate; camphor (natural); crotalus durissus terrificus venom; dibasic potassium phosphate; fucus vesiculosus; iodine; levothyroxine; liothyronine (component of)
- Adenosine triphosphate disodium; anemone pulsatilla; calcium fluoride; colchicum autumnale bulb; conium maculatum flowering top; cortisone acetate; escherichia coli; fucus vesiculosus; fumaric acid; galium aparine; iodine; lactic acid, L-; liothyronine; malic acid; mercuric chloride; oxogluric acid; phenyl isothiocyanate; pork liver; prasterone; salmonella enterica subsp. enterica serovar enteritidis; sodium diethyl oxalacetate; spongia officinalis skeleton, roasted; sus scrofa bone marrow; sus scrofa pineal gland; sus scrofa spleen; sus scrofa thymus; sus scrofa thyroid; sus scrofa umbilical cord; tyramine; viscum album fruiting top (component of)
- alpha-KETOGLUTARIC ACID; ADENOSINE TRIPHOSPHATE DISODIUM; ANEMONE PRATENSIS; CALCIUM FLUORIDE; COLCHICUM AUTUMNALE BULB; CONIUM MACULATUM FLOWERING TOP; CORTISONE ACETATE; ESCHERICHIA COLI; FUCUS VESICULOSUS; FUMARIC ACID; GALIUM APARINE; IODINE; LACTIC ACID, L-; LIOTHYRONINE; MALIC ACID; MERCURIC CHLORIDE; PHENYL ISOTHIOCYANATE; PORK LIVER; PRASTERONE; SALMONELLA ENTERICA SUBSP. ENTERICA SEROVAR ENTERITIDIS; SODIUM DIETHYL OXALACETATE; SPONGIA OFFICINALIS SKELETON, ROASTED; SUS SCROFA BONE MARROW; SUS SCROFA PINEAL GLAND; SUS SCROFA SPLEEN; SUS SCROFA THYMUS; SUS SCROFA THYROID; SUS SCROFA UMBILICAL CORD; TYRAMINE; VISCUM ALBUM FRUITING TOP (component of)
- Antimony potassium tartrate; ascorbic acid; barium carbonate; bisphenol A; bromine; chlorine; conium maculatum flowering top; cortisone acetate; echinacea angustifolia; escherichia coli; ferrum phosphoricum; galium aparine; gentiana lutea root; geranium robertianum; glyphosate; horse chestnut; hydrofluoric acid; iodine; lactic acid, L-; liothyronine; mercurius solubilis; phenylalanine; pork liver; protortonia cacti; pulsatilla vulgaris whole; salmonella enterica subsp. enterica serovar enteritidis; solanum dulcamara top; sulfur; sus scrofa adrenal gland; sus scrofa bone marrow; sus scrofa hypothalamus; sus scrofa lymph; sus scrofa spleen; sus scrofa tonsil; sus scrofa umbilical cord; tribasic calcium phosphate (component of)
- Anemone pratensis; antimony potassium tartrate; ascorbic acid; barium carbonate; bisphenol A; bromine; chlorine; conium maculatum flowering top; cortisone acetate; echinacea angustifolia; escherichia coli; ferrosoferric phosphate; galium aparine; gentiana lutea root; geranium robertianum; glyphosate; horse chestnut; hydrofluoric acid; iodine; lactic acid, L-; liothyronine; mercurius solubilis; phenylalanine; pork liver; protortonia cacti; salmonella enterica subsp. enterica serovar enteritidis; solanum dulcamara top; sulfur; sus scrofa adrenal gland; sus scrofa bone marrow; sus scrofa hypothalamus; sus scrofa lymph; sus scrofa spleen; sus scrofa tonsil; sus scrofa umbilical cord; tribasic calcium phosphate (component of)
- Antimony potassium tartrate; ascorbic acid; barium carbonate; bisphenol A; bromine; chlorine; conium maculatum flowering top; cortisone acetate; echinacea angustifolia whole; escherichia coli; ferrosoferric phosphate; galium aparine whole; gentiana lutea root; geranium robertianum whole; glyphosate; horse chestnut; hydrofluoric acid; iodine; lactic acid, L-; liothyronine; mercurius solubilis; phenylalanine; pork liver; protortonia cacti; pulsatilla pratensis whole; salmonella enterica subsp. enterica serovar enteritidis; solanum dulcamara top; sulfur; sus scrofa adrenal gland; sus scrofa bone marrow; sus scrofa hypothalamus; sus scrofa lymph; sus scrofa spleen; sus scrofa tonsil; sus scrofa umbilical cord; tribasic calcium phosphate (component of)
- Adipose Tissue
- Adrenal Gland
- Epidermis
- Fibroblasts
- Intestine
- Neuron
- Placenta
- Platelet
- Skeletal Muscle
- Testis
- Thyroid Gland
- Cytoplasm
- Extracellular
- Membrane
- Thyroid hormone synthesis
- Tyrosine metabolism

H315 (98.9%): Causes skin irritation [Warning Skin corrosion/irritation]
H319 (98.9%): Causes serious eye irritation [Warning Serious eye damage/eye irritation]
H335 (98.3%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation]
P261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 174 reports by companies from 10 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Skin Irrit. 2 (98.9%)
Eye Irrit. 2A (98.9%)
STOT SE 3 (98.3%)
IMAP assessments - L-Tyrosine, O-(4-hydroxy-3-iodophenyl)-3,5-diiodo-: Environment tier I assessment
IMAP assessments - L-Tyrosine, O-(4-hydroxy-3-iodophenyl)-3,5-diiodo-: Human health tier I assessment
M Chen, V Vijay, Q Shi, Z Liu, H Fang, W Tong. FDA-Approved Drug Labeling for the Study of Drug-Induced Liver Injury, Drug Discovery Today, 16(15-16):697-703, 2011. PMID:21624500 DOI:10.1016/j.drudis.2011.05.007
M Chen, A Suzuki, S Thakkar, K Yu, C Hu, W Tong. DILIrank: the largest reference drug list ranked by the risk for developing drug-induced liver injury in humans. Drug Discov Today 2016, 21(4): 648-653. PMID:26948801 DOI:10.1016/j.drudis.2016.02.015
◉ Summary of Use during Lactation
Liothyronine (T3) is a normal component of human milk. If replacement doses of liothyronine are required by the mother, it is not necessarily a reason to discontinue breastfeeding. However, because no information is available on the use of exogenous liothyronine during breastfeeding, an alternate drug may be preferred. The American Thyroid Association recommends that subclinical and overt hypothyroidism should be treated with levothyroxine in lactating women seeking to breastfeed. Liothyronine dosage requirement may be increased in the postpartum period compared to prepregnancy requirements patients with Hashimoto's thyroiditis.
◉ Effects in Breastfed Infants
Relevant published information was not found as of the revision date. However, the thyroid hormone content of human milk from the mothers of very preterm infants appears not to be sufficient to affect the infant’s thyroid status.
◉ Effects on Lactation and Breastmilk
Adequate thyroid hormone serum levels are required for normal lactation. Replacing deficient thyroid levels should improve milk production caused by hypothyroidism. Supraphysiologic doses of liothyronine would not be expected to further improve lactation.
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=AUYYCJSJGJYCDS-LBPRGKRZSA-N
- Australian Industrial Chemicals Introduction Scheme (AICIS)L-Tyrosine, O-(4-hydroxy-3-iodophenyl)-3,5-diiodo-https://services.industrialchemicals.gov.au/search-assessments/
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/L-Tyrosine, O-(4-hydroxy-3-iodophenyl)-3,5-diiodo-, labeled with iodine-125https://commonchemistry.cas.org/detail?cas_rn=15785-49-6Triiodothyroninehttps://commonchemistry.cas.org/detail?cas_rn=6893-02-3
- ChemIDplusLiothyronine [INN:BAN]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0006893023ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_useLiothyroninehttps://www.drugbank.ca/drugs/DB00279
- EPA DSSTox3,5,3'-Triiodothyroninehttps://comptox.epa.gov/dashboard/DTXSID8023216CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeLiothyronine (EC: 229-999-3)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/38650
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Hazardous Substances Data Bank (HSDB)
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingLiothyroninehttp://www.hmdb.ca/metabolites/HMDB0000265HMDB0000265_msms_3846https://hmdb.ca/metabolites/HMDB0000265#spectra
- BindingDBLICENSEAll data curated by BindingDB staff are provided under the Creative Commons Attribution 3.0 License (https://creativecommons.org/licenses/by/3.0/us/).https://www.bindingdb.org/rwd/bind/info.jsp(2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-diiodophenyl]propanoic acidhttps://www.bindingdb.org/rwd/bind/chemsearch/marvin/MolStructure.jsp?monomerid=18860
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jspTriiodothyroninehttps://ctdbase.org/detail.go?type=chem&acc=D014284
- Drug Gene Interaction database (DGIdb)LICENSEThe data used in DGIdb is all open access and where possible made available as raw data dumps in the downloads section.http://www.dgidb.org/downloadsLIOTHYRONINEhttps://www.dgidb.org/drugs/rxcui:10814
- IUPHAR/BPS Guide to PHARMACOLOGYLICENSEThe Guide to PHARMACOLOGY database is licensed under the Open Data Commons Open Database License (ODbL) https://opendatacommons.org/licenses/odbl/. Its contents are licensed under a Creative Commons Attribution-ShareAlike 4.0 International License (http://creativecommons.org/licenses/by-sa/4.0/)https://www.guidetopharmacology.org/about.jsp#licenseGuide to Pharmacology Target Classificationhttps://www.guidetopharmacology.org/targets.jsp
- Therapeutic Target Database (TTD)Liothyroninehttps://idrblab.net/ttd/data/drug/details/D0S6JGTriiodothyroninehttps://idrblab.net/ttd/data/drug/details/D07NPX
- Toxin and Toxin Target Database (T3DB)LICENSET3DB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (T3DB) and the original publication.http://www.t3db.ca/downloadsLiothyroninehttp://www.t3db.ca/toxins/T3D4759
- Burnham Center for Chemical Genomics
- CCSbaseCCSbase Classificationhttps://ccsbase.net/
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/LiothyronineNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- ChEBI3,3',5-triiodo-L-thyroninehttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:18258
- E. coli Metabolome Database (ECMDB)
- FDA Pharm ClassesLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingFDA Pharmacological Classificationhttps://www.fda.gov/ForIndustry/DataStandards/StructuredProductLabeling/ucm162549.htm
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/Liothyroninehttps://www.wikidata.org/wiki/Q327362LOTUS Treehttps://lotus.naturalproducts.net/
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licenceLIOTHYRONINEhttps://platform.opentargets.org/drug/CHEMBL1544
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- DailyMed
- Drug Induced Liver Injury Rank (DILIrank) DatasetLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Drugs and Lactation Database (LactMed)
- ECI Group, LCSB, University of LuxembourgTriiodothyronine
- Natural Product Activity and Species Source (NPASS)
- EU Clinical Trials Register
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-law3,3',5-Triiodo-L-thyroninehttp://www.nist.gov/srd/nist1a.cfm
- SpectraBase2,2'-Azinobis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salthttps://spectrabase.com/spectrum/16pAcismm6dL-3,3',5-Triiodothyroninehttps://spectrabase.com/spectrum/2zEmm6wlNFnL-Triiodothyroninehttps://spectrabase.com/spectrum/34hWkLyjhrIL-Triodothyroninehttps://spectrabase.com/spectrum/LrqVKtK6vN52,2'-Azinobis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salthttps://spectrabase.com/spectrum/GqbxcH6zzq9
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlCompounds with biological roleshttp://www.genome.jp/kegg-bin/get_htext?br08001.kegTarget-based classification of drugshttp://www.genome.jp/kegg-bin/get_htext?br08310.keg
- MarkerDBLICENSEThis work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.https://markerdb.ca/Liothyroninehttps://markerdb.ca/chemicals/173
- MassBank Europe
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- Metabolomics Workbench
- National Drug Code (NDC) DirectoryLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Nature Chemical Biology
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.htmlliothyroninehttps://rxnav.nlm.nih.gov/id/rxnorm/10814
- NMRShiftDB
- PharmGKBLICENSEPharmGKB data are subject to the Creative Commons Attribution-ShareALike 4.0 license (https://creativecommons.org/licenses/by-sa/4.0/).https://www.pharmgkb.org/page/policiesliothyroninehttps://www.pharmgkb.org/chemical/PA164778866
- PharosLICENSEData accessed from Pharos and TCRD is publicly available from the primary sources listed above. Please respect their individual licenses regarding proper use and redistribution.https://pharos.nih.gov/aboutliothyroninehttps://pharos.nih.gov/ligands/3XYSNU34MN46
- Protein Data Bank in Europe (PDBe)
- RCSB Protein Data Bank (RCSB PDB)LICENSEData files contained in the PDB archive (ftp://ftp.wwpdb.org) are free of all copyright restrictions and made fully and freely available for both non-commercial and commercial use. Users of the data should attribute the original authors of that structural data.https://www.rcsb.org/pages/policies
- Springer Nature
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidata3,3',5-triiodo-L-thyronine zwitterionhttps://www.wikidata.org/wiki/Q106345628
- WikipediaTriiodothyroninehttps://en.wikipedia.org/wiki/TriiodothyronineLiothyroninehttps://en.wikipedia.org/wiki/Liothyronine
- Wiley
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlTriiodothyroninehttps://www.ncbi.nlm.nih.gov/mesh/68014284
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403383321https://pubchem.ncbi.nlm.nih.gov/substance/403383321
- NCBI








