Ketorolac Tromethamine
- Ketorolac tromethamine
- 74103-07-4
- Ketorolac tris salt
- Toradol
- Acular
- Create:2005-06-24
- Modify:2025-01-04
- Acular
- Ketorolac Tromethamine
- Toradol
- Ketorolac tromethamine
- 74103-07-4
- Ketorolac tris salt
- Toradol
- Acular
- Ketorolac tromethamine salt
- Ketorolac (tromethamine salt)
- Acuvail
- Lixidol
- Ketorolac trometamol
- Acular LS
- Acular PF
- Syntex
- Dolac
- SPRIX
- Tarasyn
- Godek
- Acular Preservative Free
- Tromethamine ketorolac
- 2-amino-2-(hydroxymethyl)propane-1,3-diol 5-benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylate
- UNII-4EVE5946BQ
- NSC-758637
- Ketorolac (Toradol)
- 4EVE5946BQ
- DTXSID0045597
- MFCD00887595
- CHEBI:6130
- DTXCID301476271
- Ketorolac tromethamine [USAN:USP]
- NSC 758637
- 2-amino-2-(hydroxymethyl)propane-1,3-diol; 5-benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid
- Toradol (TN)
- OMIDRIA COMPONENT KETOROLAC TROMETHAMINE
- Ketorolac tromethamine (USAN:USP)
- KETOROLAC TROMETAMOL (MART.)
- KETOROLAC TROMETAMOL [MART.]
- Ketorolac Tromethamine;Ketorolac tris salt;RS37619 tromethamine salt
- (+/-)-5-Benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid tris salt
- KETOROLAC TROMETHAMINE (USP-RS)
- KETOROLAC TROMETHAMINE [USP-RS]
- (+/-)-5-BENZOYL-2,3-DIHYDRO-1H-PYRROLIZINE-1-CARBOXYLIC ACID, COMPOUND WITH 2-AMINO-2-(HYDROXYMETHYL)-1,3-PROPANEDIOL (1:1)
- 1H-PYRROLIZINE-1-1H-PYRROLIZINE-1-CARBOXYLIC ACID, 5-BENZOYL-2,3-DIHYDRO, (+/-)-, COMPOUND WITH 2-AMINO-2-(HYDROXYMETHYL)-1,3-PROPANEDIOL (1:1)
- 2-AMINO-2-(HYDROXYMETHYL)PROPANE-1,3-DIOL (1RS)-5-BENZOYL-2,3-DIHYDRO-1H-PYRROLIZINE-1-CARBOXYLATE
- SMR000058461
- KETOROLAC TROMETAMOL (EP MONOGRAPH)
- KETOROLAC TROMETAMOL [EP MONOGRAPH]
- Ketorolac tromethamine [USAN]
- KETOROLAC TROMETHAMINE (USP MONOGRAPH)
- KETOROLAC TROMETHAMINE [USP MONOGRAPH]
- SR-01000075948
- NCGC00185990-01
- RS-37619
- Toratex
- Droal
- Ketorolactrissalt
- Tora-Dol
- Toronova SUIK
- Acunivive 15
- Acunivive 30
- Acunivive 60
- NCGC00017135-01
- Toronova II SUIK
- ReadySharp Ketorolac
- Acular (TN)
- CAS-74103-07-4
- BPPC
- Ketotolac Tromethamine
- Spectrum_001578
- CPD000058461
- 1,3-dihydroxy-2-(hydroxymethyl)propan-2-aminium 5-benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylate
- Prestwick0_000929
- Prestwick1_000929
- Prestwick2_000929
- Prestwick3_000929
- Spectrum2_001598
- Spectrum3_001975
- Spectrum4_000215
- Spectrum5_001273
- Ketrolac.Tromethamine Salt
- SCHEMBL5036
- Lopac0_000676
- BSPBio_000838
- BSPBio_003575
- KBioGR_000849
- KBioSS_002058
- (+-)-5-Benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid, compound with 2-amino-2-(hydroxymethyl)-1,3-propanediol (1:1)
- 1H-Pyrrolizine-1-carboxylic acid, 5-benzoyl-2,3-dihydro, (+/-)-, compound with 2-amino-2-(hydroxymethyl)-1,3-propanediol(1:1)
- Ketorolac tromethamine (USP)
- MLS000069689
- MLS001401455
- MLS002222310
- DivK1c_000836
- SPECTRUM1503925
- SPBio_001596
- SPBio_003017
- BPBio1_000922
- CHEMBL1201124
- HMS502J18
- HY-B0138R
- KBio1_000836
- KBio2_002058
- KBio2_004626
- KBio2_007194
- KBio3_002953
- ROX-828
- ROX-888
- NINDS_000836
- HMS1570J20
- HMS1922K22
- HMS2051P06
- HMS2093M05
- HMS2097J20
- HMS2235N13
- HMS3262G13
- HMS3373M06
- HMS3393P06
- HMS3655E15
- HMS3714J20
- KETOROLAC TROMETAMOL [JAN]
- Pharmakon1600-01503925
- BCP02917
- HY-B0138
- Tox21_110795
- Tox21_113519
- Tox21_500676
- CCG-39376
- NSC758637
- s5698
- KETOROLAC TROMETHAMINE [VANDF]
- AKOS024386301
- Tox21_110795_1
- BCP9000811
- CCG-101006
- CS-1933
- KETOROLAC TROMETHAMINE [WHO-DD]
- LP00676
- NC00256
- IDI1_000836
- NCGC00016159-02
- NCGC00016159-03
- NCGC00016159-04
- NCGC00094036-01
- NCGC00094036-02
- NCGC00094036-03
- NCGC00185990-04
- NCGC00261361-01
- 1H-Pyrrolizine-1-carboxylic acid, 5-benzoyl-2,3-dihydro, (+-)-, compound with 2-amino-2-(hydroxymethyl)-1,3-propanediol (1:1)
- 2-amino-2-(hydroxymethyl)propane-1,3-diol;5-benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid
- AS-15193
- Ketorolac (tromethamine salt) (Standard)
- Ketorolac tris salt;Ketorolac Tromethamine
- BCP0726000301
- Ketorolac tris salt, >=99%, crystalline
- DB-055837
- Ketorolac trometamol for peak identification
- KETOROLAC TROMETHAMINE [ORANGE BOOK]
- EU-0100676
- K0053
- SW197293-4
- D00813
- K 1136
- M02042
- KETOROLAC TROMETHAMINE COMPONENT OF OMIDRIA
- SR-01000946595
- Q-201269
- SR-01000075948-1
- SR-01000075948-6
- SR-01000075948-8
- SR-01000946595-1
- KETOROLAC (+/-)-FORM TROMETHAMINE SALT [MI]
- Q27107089
- F0001-2390
- Ketorolac Trometamol 1.0 mg/ml in Methanol (as free acid)
- Ketorolac trometamol, European Pharmacopoeia (EP) Reference Standard
- Ketorolac Tromethamine, United States Pharmacopeia (USP) Reference Standard
- Ketorolac trometamol for peak identification, European Pharmacopoeia (EP) Reference Standard
- Ketorolac Tromethamine, Pharmaceutical Secondary Standard; Certified Reference Material
256.098053 100
105.034317 36.94
257.100800 10.73
178.052170 5.51
106.065903 3.17
Ketorolac (has active moiety)
- Ketorolac tromethamine; phenylephrine hydrochloride (component of)
- Bupivacaine hydrochloride; chitosan low molecular weight (20-200 mpa.S); ketorolac tromethamine (component of)


H301 (96.9%): Toxic if swallowed [Danger Acute toxicity, oral]
H315 (93.8%): Causes skin irritation [Warning Skin corrosion/irritation]
H319 (93.8%): Causes serious eye irritation [Warning Serious eye damage/eye irritation]
H335 (92.2%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation]
P261, P264, P264+P265, P270, P271, P280, P301+P316, P302+P352, P304+P340, P305+P351+P338, P319, P321, P330, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 64 reports by companies from 12 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Acute Tox. 3 (96.9%)
Skin Irrit. 2 (93.8%)
Eye Irrit. 2 (93.8%)
STOT SE 3 (92.2%)
◉ Summary of Use during Lactation
Milk levels of ketorolac are low with the usual oral dosage, but milk levels have not been measured after higher injectable dosages or with the nasal spray. Ketorolac injection is used for a short time (typically 24 hours) after cesarean section in some hospital protocols with no evidence of harm to breastfed infants. However, the ketorolac dose an infant receives in colostrum is very low because of the small volume of colostrum produced. Some evidence suggests that IV ketorolac as part of a multimodal post-cesarean section analgesia reduces percentage of mothers who fail exclusive breastfeeding compared to patient-controlled IV morphine-based analgesia. Ketorolac has strong antiplatelet activity and can cause gastrointestinal bleeding. The manufacturer indicates that ketorolac is contraindicated during breastfeeding, so an alternate drug is preferred after the first 24 to 72 hours when larger volumes of milk are produced, especially while nursing a newborn or preterm infant.
Maternal use of ketorolac eye drops would not be expected to cause any adverse effects in breastfed infants. To substantially diminish the amount of drug that reaches the breastmilk after using eye drops, place pressure over the tear duct by the corner of the eye for 1 minute or more, then remove the excess solution with an absorbent tissue.
◉ Effects in Breastfed Infants
A randomized, double-blind study compared standard care of mothers receiving a cesarean section delivery (n = 60) to those receiving standard care plus multimodal pain management that included a single dose of 60 mg of intramuscular ketorolac given at the time of fascial closure (n = 60). No significant differences in abnormal neonatal growth, difficulty feeding, neonatal sedation, or respiratory depression rates between the two groups were seen during the first month postpartum.
◉ Effects on Lactation and Breastmilk
A randomized, double-blind study compared standard care of mothers receiving a cesarean section delivery (n = 60) to those receiving standard care plus multimodal pain management that included a single dose of 60 mg of intramuscular ketorolac given at the time of fascial closure (n = 60). No significant differences in breastfeeding rates (78% and 79%, respectively) were seen during the first month postpartum.
In a study comparing standard of care to enhanced recovery after cesarean section deliveries, a fixed dose of ketorolac 15 mg every 6 hours intravenously for 24 hours postpartum was part of the enhanced recovery protocol whereas as needed ketorolac 15 mg intravenously was part of the standard protocol. Patients in the enhanced recovery protocol (n = 58) had a greater frequency of exclusive breastfeeding (67%) than those in the standard protocol (48%; n = 60).
A retrospective study evaluated 1349 women who had undergone a cesarean section and were given ketorolac within 15 minutes of the end of surgery. The results indicated that there was no difference in pain control in the first 6 hours after surgery nor in the percentage of women who were breastfeeding at discharge.
A prospective cohort study of postcesarean pain control compared (1) morphine PCA and scheduled ibuprofen for the first 12 hours followed by continued scheduled ibuprofen with hydrocodone-acetaminophen as needed to a multimodal pain management regimen consisting of (2) acetaminophen 1000 mg orally every 8 hours, ketorolac 30 mg IV once initially, then 15 mg IV every 8 hours for 24 hours, then ibuprofen 600 mg orally every 8 hours for the remainder of the postoperative course with opioids given only as needed. Of women who planned to exclusively breastfeed on admission, fewer women used formula prior to discharge in the multimodal group compared to the traditional group (9% vs. 12%).
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=BWHLPLXXIDYSNW-UHFFFAOYSA-N
- Burnham Center for Chemical Genomics
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/Ketorolac tromethaminehttps://commonchemistry.cas.org/detail?cas_rn=74103-07-4
- ChemIDplusKetorolac tromethamine [USAN:USP]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0074103074ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DTP/NCILICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuse
- EPA DSSToxKetorolac tromethaminehttps://comptox.epa.gov/dashboard/DTXSID0045597CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-notice(±)-5-benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid,2-amino-2-(hydroxymethyl)-1,3-propanediolhttps://echa.europa.eu/substance-information/-/substanceinfo/100.149.467(±)-5-benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid,2-amino-2-(hydroxymethyl)-1,3-propanediol (EC: 620-545-3)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/154008
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingKETOROLAC TROMETHAMINEhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/4EVE5946BQ
- CCSbaseCCSbase Classificationhttps://ccsbase.net/
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jspKetorolac Tromethaminehttps://ctdbase.org/detail.go?type=chem&acc=D020911
- Drug Gene Interaction database (DGIdb)LICENSEThe data used in DGIdb is all open access and where possible made available as raw data dumps in the downloads section.http://www.dgidb.org/downloadsKETOROLAC TROMETHAMINEhttps://www.dgidb.org/drugs/rxcui:28200
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licenceKETOROLAC TROMETHAMINEhttps://platform.opentargets.org/drug/CHEMBL1201124
- DailyMed
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_use
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- Drugs and Lactation Database (LactMed)
- Drugs@FDALICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- EU Clinical Trials Register
- FDA Medication GuidesLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- FDA Orange BookLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlUSP drug classificationhttp://www.genome.jp/kegg-bin/get_htext?br08302.kegAnatomical Therapeutic Chemical (ATC) classificationhttp://www.genome.jp/kegg-bin/get_htext?br08303.kegTarget-based classification of drugshttp://www.genome.jp/kegg-bin/get_htext?br08310.keg
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- National Drug Code (NDC) DirectoryLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingKETOROLAC TROMETHAMINEhttps://www.fda.gov/drugs/drug-approvals-and-databases/national-drug-code-directory
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.htmlketorolac tromethaminehttps://rxnav.nlm.nih.gov/id/rxnorm/28200
- SpectraBaseKetorolac tromethaminehttps://spectrabase.com/spectrum/1WllfPWDXmZKetorolac tromethaminehttps://spectrabase.com/spectrum/EkL8c30niKl
- Springer Nature
- Wikidataketorolac tromethaminehttps://www.wikidata.org/wiki/Q27107089
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlKetorolac Tromethaminehttps://www.ncbi.nlm.nih.gov/mesh/68020911Anti-Inflammatory Agents, Non-Steroidalhttps://www.ncbi.nlm.nih.gov/mesh/68000894Cyclooxygenase Inhibitorshttps://www.ncbi.nlm.nih.gov/mesh/68016861
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403614081https://pubchem.ncbi.nlm.nih.gov/substance/403614081
- NCBI
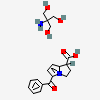

 CID 6503 (Tromethamine)
CID 6503 (Tromethamine)