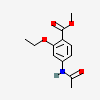
Ethopabate
- ETHOPABATE
- Methyl 4-acetamido-2-ethoxybenzoate
- 59-06-3
- Ethopabat
- Benzoic acid, 4-(acetylamino)-2-ethoxy-, methyl ester
- Create:2005-07-19
- Modify:2025-01-11
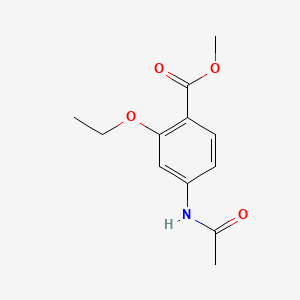
- ETHOPABATE
- Methyl 4-acetamido-2-ethoxybenzoate
- 59-06-3
- Ethopabat
- Benzoic acid, 4-(acetylamino)-2-ethoxy-, methyl ester
- EINECS 200-414-3
- MFCD00075809
- UNII-F4X3L6068O
- Methyl 4-(acetylamino)-2-ethoxybenzoate
- DTXSID6046264
- 4-Acetamido-2-ethoxybenzoesaeuremethylester
- F4X3L6068O
- Ethopabate [USP:BAN]
- DTXCID4026264
- NCGC00160624-01
- Ethopabate 100 microg/mL in Acetonitrile
- Ethopabate (USP:BAN)
- Ethyl Pabate
- ETHOPABATE (MART.)
- ETHOPABATE [MART.]
- ETHOPABATE (USP-RS)
- ETHOPABATE [USP-RS]
- ETHOPABATE (USP MONOGRAPH)
- ETHOPABATE [USP MONOGRAPH]
- CAS-59-06-3
- Amprol Plus (veterinary)
- Methyl4-acetamido-2-ethoxybenzoate
- Ethopabate (USP)
- Methyl 2-ethoxy-4-acetamidobenzoate
- Ethopabate (Standard)
- ETHOPABATE [MI]
- 4-acetylamino-2-ethoxybenzoic acid methyl ester
- SCHEMBL540932
- CHEMBL458769
- ETHOPABATE [GREEN BOOK]
- HY-B2138R
- CHEBI:183844
- HY-B2138
- Tox21 111943
- Tox21_111943
- s3707
- Methyl 4-acetamido-2-ethoxy-benzoate
- AKOS015892662
- Tox21_111943_1
- CCG-213430
- CS-8028
- NCGC00160624-02
- NCGC00160624-03
- NCGC00160624-04
- AS-11898
- SY226309
- DB-053312
- NS00000013
- 4-Acetamido-2-ethoxybenzoic acid, methyl ester
- D08916
- Ethopabate, VETRANAL(TM), analytical standard
- AB01563313_01
- 4-Acetylamino-2-ethoxy-benzoic acid, methyl ester
- SR-01000944203
- Q-201078
- Q5404419
- SR-01000944203-1
- BRD-K02475039-001-01-0
- Ethopabate, British Pharmacopoeia (BP) Reference Standard
- Ethopabate, United States Pharmacopeia (USP) Reference Standard
206.0807 999
238.1071 106
207.084 79
239.1118 8
164.0695 7
206.0809 999
164.0701 585
136.0752 150
136.0386 128
178.0494 117
- Amprolium; Ethopabate (component of)
- Amprolium; Bacitracin Zinc; Ethopabate (component of)
- Amprolium; Bambermycins; Ethopabate (component of)
- Amprolium; Chlortetracycline; Ethopabate (component of)
- Amprolium; Bacitracin methylenedisalicylate; Ethopabate (component of)

H302 (95.7%): Harmful if swallowed [Warning Acute toxicity, oral]
H319 (85.1%): Causes serious eye irritation [Warning Serious eye damage/eye irritation]
P264, P264+P265, P270, P280, P301+P317, P305+P351+P338, P330, P337+P317, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 47 reports by companies from 4 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Reported as not meeting GHS hazard criteria per 1 of 47 reports by companies. For more detailed information, please visit ECHA C&L website.
There are 3 notifications provided by 46 of 47 reports by companies with hazard statement code(s).
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Acute Tox. 4 (95.7%)
Eye Irrit. 2 (85.1%)
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=GOVWOKSKFSBNGD-UHFFFAOYSA-N
- Australian Industrial Chemicals Introduction Scheme (AICIS)Benzoic acid, 4-(acetylamino)-2-ethoxy-, methyl esterhttps://services.industrialchemicals.gov.au/search-inventory/
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusEthopabate [USP:BAN]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000059063ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxCompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeMethyl 4-acetamido-2-ethoxybenzoatehttps://echa.europa.eu/substance-information/-/substanceinfo/100.000.377Methyl 4-acetamido-2-ethoxybenzoate (EC: 200-414-3)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/47559
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- ChEBI
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- Crystallography Open Database (COD)LICENSEAll data in the COD and the database itself are dedicated to the public domain and licensed under the CC0 License. Users of the data should acknowledge the original authors of the structural data.https://creativecommons.org/publicdomain/zero/1.0/
- The Cambridge Structural Database
- FDA Approved Animal Drug Products (Green Book)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/EthopabateNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.html
- MassBank Europe
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawMethyl 4-(acetylamino)-2-ethoxybenzoatehttp://www.nist.gov/srd/nist1a.cfm
- SpectraBase
- Springer Nature
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidataethopabatehttps://www.wikidata.org/wiki/Q5404419
- WikipediaNitronium perchloratehttps://en.wikipedia.org/wiki/Nitronium_perchlorateEthopabatehttps://en.wikipedia.org/wiki/Ethopabate
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlCoccidiostatshttps://www.ncbi.nlm.nih.gov/mesh/68003049
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403462085https://pubchem.ncbi.nlm.nih.gov/substance/403462085