Dipyrone
- dipyrone
- Analgin
- Metamizole sodium
- 68-89-3
- Novalgin
- Create:2005-03-27
- Modify:2024-12-14
- Algopyrin
- Analgin
- Biopyrin
- Dipyrone
- Dipyronium
- Metamizol
- Metamizole
- Metamizole Sodium
- Methamizole
- Methampyrone
- Narone
- Noramidopyrine Methanesulfonate
- Noramidopyrine Methanesulfonate Sodium
- Normelubrine
- Novalgetol
- Novalgin
- Novamidazophen
- Novaminsulfone
- Optalgin
- Pyralgin
- Sulpyrin
- Sulpyrine
- dipyrone
- Analgin
- Metamizole sodium
- 68-89-3
- Novalgin
- Methampyrone
- Algocalmin
- Methylmelubrin
- Neomelurbrin
- sulpyrine
- Novalgetol
- Optalgin
- Pyralgin
- Sulpyrin
- Fevonil
- Narone
- Novamidazophen
- Novaminsulfone
- Alginodia
- Algopyrine
- Bonpyrin
- Dimethone
- Diprofarn
- Farmolisina
- Feverall
- Keypyrone
- Metapyrin
- Nevralgina
- Noveltex
- Paralgin
- Pharmalgine
- Barone
- Conmel
- Gifaril
- Metilon
- Pyretin
- Pyrojec
- Sulpin
- Neo-melubrine
- Di-podil
- ARPF
- Novaminsulfon
- Novaminsulfonum
- Sulpyrinum
- Analgine
- Analginum
- Aminopyrine sodium sulfonate
- Metamizolnatrium
- Metamizolum natricum
- Novaminsulfonium
- Novaminophenazone
- Metamizolo
- Novamidazophenum
- Novaminsulfone sodium
- Meamizol sodico
- Noraminophenazone
- Noraminophenazone sodium mesylate
- Sodium novaminsulfonate
- Dipyrone anhydrous
- Metamizole sodique
- Pyretin (pharmaceutical)
- Noramidopyrine methanesulfonate sodium
- Sodium noramidopyrine methanesulfonate
- Prodolina
- Vetalgin
- Mexican aspirin
- Metamizol sodico
- Metamizolo sodico
- Natrium novaminsulfonicum
- Methamizole sodium
- Sodium methylaminoantipyrine methanesulfonate
- Noraminophenazone methanesulfonate sodium salt
- Dipyrone [BAN]
- Sodium (antipyrinylmethylamino)methanesulfonate
- Noramidopyrine methanesulfonate sodium salt
- Metamizolum natricum [Latin]
- Methylaminoantipyrine sodium methanesulfonate
- Noraminopyrine methanesulfonate sodium
- Analgin (sodium salt)
- CCRIS 4443
- CHEBI:59033
- Metamizol sodium
- Metamizole sodique [INN-French]
- UNII-VSU62Z74ON
- EINECS 200-694-7
- VSU62Z74ON
- Methylaminophenyldimethylpyrazolone methanesulfonate sodium
- 4-Sodium methanesulfonate methylamine-antipyrine
- Metamizole (sodium)
- Noramidopyrinium-methanesulphate natrium
- Methanesulfonic acid, (antipyrinylmethylamino)-, sodium salt
- Dipyrone (anhydrous)
- Dipyrone [anhydrous]
- Dipyrone hydrate
- DTXSID8020543
- Metamizole Sodium Salt
- (Antipyrinylmethylamino)methanesulfonic acid sodium salt
- Sodium 1-phenyl-2,3-dimethyl-4-methylaminopyrazolon-N-methanesulfonate
- Antipyrine, 4-(methylamino)-, monomethosulfate, sodium salt
- Metamizole sodium [INN]
- Sodium phenyldimethylpyrazolon-methylamino-methane sulfonate
- CCRIS 272
- Phenyl dimethyl pyrazolon methyl aminomethane sodium sulfonate
- Methanesulfonic acid, ((2,3-dihydro-1,5-dimethyl-3-oxo-2-phenyl-1H-pyrazol-4-yl)methylamino)-, sodium salt
- Noramidopyrine Methanesulphonate Sodium
- 68-89-3 (sodium)
- Fenildimetil-pirazolon-metilaminometansolfonato sodico
- 1-Phenyl-2,3-dimethyl-5-pyrazolone-4-methylaminomethanesulfonate sodium
- 4-Methylamino-1,5-dimethyl-2-phenyl-3-pyrazolone sodium methanesulfonate
- Sodium 1-phenyl-2,3-dimethyl-5-pyrazolone-4-methylamino methanesulfonate
- Sodium 4-methylamino-1,5-dimethyl-2-phenyl-3-pyrazolone 4-methanesulfonate
- Metamizole sodium (anhydrous)
- 1-Phenyl-2,3-dimethylpyrazolone-(5)-4-methylaminomethanesulfonic acid sodium
- DTXCID20543
- Metamizolum natricum anhydricum
- sodium [(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)(methyl)amino]methanesulfonate
- Methanesulfonic acid, (antipyrinylmethylamino)-, monosodium salt
- Dipyrone (BAN)
- 1-Phenyl-2,3-dimethyl-4-methylamino-5-pyrazolon-N-methanesulfonsaeuren natrium
- UNII-6429L0L52Y
- NCGC00017049-01
- Metamizolo [Italian]
- Dipyron monohydrate
- sodium;[(1,5-dimethyl-3-oxo-2-phenylpyrazol-4-yl)-methylamino]methanesulfonate
- Sodium ((2,3-dihydro-1,5-dimethyl-3-oxo-2-phenyl-1H-pyrazol-4-yl)methylamino)methanesulphonate
- Metamizolum natricum (Latin)
- Metamizolo sodico [DCIT]
- Sodium ((1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)(methyl)amino)methanesulfonate
- Metamizol sodico [Spanish]
- (Antipyrinylmethylamino)methanesulfonic acid sodium salt monohydrate
- Metamizole sodique (INN-French)
- Methanesulfonic acid, (antipyrinylmethylamino)-, sodium salt, monohydrate
- Metamizolum natricum [INN-Latin]
- METAMIZOLE SODIUM (EP IMPURITY)
- METAMIZOLE SODIUM [EP IMPURITY]
- CAS-68-89-3
- SODIUM 1-PHENYL-2,3-DIMETHYL-4-METHYLAMINOPYRAZOLON-N-METHANESULPHONATE
- Metamizole sodique [French]
- metamizole sodium salt monohydrate
- Methanesulfonic acid, [(2,3-dihydro-1,5-dimethyl-3-oxo-2-phenyl-1H-pyrazol-4-yl)methylamino]-, sodium salt
- NSC-758445
- Dipyrone [USAN:JAN]
- Methylmelubrine
- dipyrone sodium
- METAMIZOLE SODIUM MONOHYDRATE (EP MONOGRAPH)
- Noramidopyrinium-methanesulphate natrium [German]
- Dipyrone,(S)
- Vetalgin (TN)
- Prestwick_987
- MFCD00020783
- SODIUM (ANTIPYRINYLMETHYLAMINO)METHANESULPHONATE MONOHYDRATE
- Fenildimetil-pirazolon-metilaminometansolfonato sodico [Italian]
- ((2,3-DIHYDRO-1,5-DIMETHYL-3-OXO-2-PHENYL-1H-PYRAZOL-4-YL)METHYLAMINO)METHANESULFONIC ACID SODIUM SALT MONOHYDRATE
- 54017-59-3
- Epitope ID:124939
- 1-Phenyl-2,3-dimethyl-4-methylamino-5-pyrazolon-N-methanesulfonsaeuren natrium [German]
- SCHEMBL24996
- Dipyrone [USAN:BAN:JAN]
- SPECTRUM1503298
- CHEMBL487894
- Metamizol sodico [INN-Spanish]
- HMS501P16
- HY-B1279A
- HMS1570O07
- HMS1922A06
- HMS2093C17
- HMS2097O07
- HMS3714O07
- METAMIZOLE SODIUM [WHO-DD]
- NSC73205
- Tox21_110759
- Tox21_200497
- CCG-39331
- AKOS015904648
- Tox21_110759_1
- NCGC00017049-02
- NCGC00017049-03
- NCGC00095040-01
- NCGC00095040-02
- NCGC00178220-05
- NCGC00258051-01
- AS-81983
- CAS-5907-38-0
- DB-231787
- CS-0013577
- M3060
- NS00021332
- D08190
- F20711
- Q422761
- Methanesulfonic acid, 1-[(2,3-dihydro-1,5-dimethyl-3-oxo-2-phenyl-1H-pyrazol-4-yl)methylamino]-, sodium salt (1:1)
56 99.99
83 26.30
42 21.80
217 18.70
123 15
56 99.99
217 78
123 72
83 65
215 52
56 999
83 263
42 218
217 187
123 150
56 999
217 780
123 720
83 650
215 520



H302 (11.6%): Harmful if swallowed [Warning Acute toxicity, oral]
H317 (49.5%): May cause an allergic skin reaction [Warning Sensitization, Skin]
H334 (49.5%): May cause allergy or asthma symptoms or breathing difficulties if inhaled [Danger Sensitization, respiratory]
H361 (30.5%): Suspected of damaging fertility or the unborn child [Warning Reproductive toxicity]
H372 (29.5%): Causes damage to organs through prolonged or repeated exposure [Danger Specific target organ toxicity, repeated exposure]
H411 (29.5%): Toxic to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard]
P203, P233, P260, P261, P264, P270, P271, P272, P273, P280, P284, P301+P317, P302+P352, P304+P340, P318, P319, P321, P330, P333+P317, P342+P316, P362+P364, P391, P403, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 95 reports by companies from 10 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Reported as not meeting GHS hazard criteria per 4 of 95 reports by companies. For more detailed information, please visit ECHA C&L website.
There are 9 notifications provided by 91 of 95 reports by companies with hazard statement code(s).
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Acute Tox. 4 (11.6%)
Skin Sens. 1 (49.5%)
Resp. Sens. 1 (49.5%)
Repr. 2 (30.5%)
STOT RE 1 (29.5%)
Aquatic Chronic 2 (29.5%)
IMAP assessments - Methanesulfonic acid, [(2,3-dihydro-1,5-dimethyl-3-oxo-2-phenyl-1H-pyrazol-4-yl)methylamino]-, sodium salt: Environment tier I assessment
IMAP assessments - Methanesulfonic acid, [(2,3-dihydro-1,5-dimethyl-3-oxo-2-phenyl-1H-pyrazol-4-yl)methylamino]-, sodium salt: Human health tier I assessment
◉ Summary of Use during Lactation
After ingestion by the mother, dipyrone and its metabolites appear in breastmilk in rather large amounts. It is found in the blood and urine of breastfed infants and can cause pharmacological effects in the breastfed infant. One case of cyanotic episodes in a breastfed infant was attributed to dipyrone in breastmilk. The drug and metabolites are eliminated from the breastmilk by 48 hours after a dose.
Dipyrone is not approved for marketing in the United States by the U.S. Food and Drug Administration nor in Canada and many European countries because of its adverse reactions, including agranulocytosis. However, it is widely used in other countries during labor and breastfeeding. The European Medicines Agency recommends that dipyrone not be used in nursing mothers; however, several drug consultation centers in Israel disagree. One manufacturer recommends to withhold breastfeeding for 48 hours after a dose. Safer alternatives are available for analgesia during breastfeeding.
◉ Effects in Breastfed Infants
A 42-day-old breastfed infant had 2 cyanotic episodes within 30 minutes after his mother took 3 doses of dipyrone 500 mg orally, 18, 7 and 2 hours before the first episode. A third episode occurred 24 hours after admission to the hospital. Dipyrone was detected in the mother's breastmilk 24 hours after the last dose and in the infant's serum and urine. No explanation could be found for the cyanotic episodes other than dipyrone and after suspending maternal dipyrone intake, no further episodes occurred in the infant up to age 3 years. The reaction is rated as possibly caused by dipyrone in breastmilk.
In a blinded study, mothers who were at least 3 days postpartum and requesting analgesia for postpartum uterine pain were given either 1 gram of dipyrone or placebo. The infants of mothers who received dipyrone cried fewer times and for shorter durations in the 14 hours after drug administration than the infants of mothers who received placebo. This effect was more apparent in infants who demand fed than in those who fed on a fixed schedule. Although this study appears to demonstrate a pharmacologic effect in the infants from dipyrone in milk, there is no clear explanation for the change in infant behavior.
A multicenter case-control study in Brazil compared 231 children who developed leukemia before 2 years of age with 411 children with various other nonmalignant diseases. Mothers were interviewed to ascertain their analgesic use during pregnancy and lactation. Nursing mothers who took dipyrone during the three months after delivery had a 2-fold risk of having a child with acute lymphocytic leukemia and a 3.87-fold risk in having rearrangement of the MLL gene in infants under one year of age.
◉ Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=DJGAAPFSPWAYTJ-UHFFFAOYSA-M
- Australian Industrial Chemicals Introduction Scheme (AICIS)Methanesulfonic acid, [(2,3-dihydro-1,5-dimethyl-3-oxo-2-phenyl-1H-pyrazol-4-yl)methylamino]-, sodium salthttps://services.industrialchemicals.gov.au/search-assessments/
- ChemIDplusDipyrone [USAN:BAN:JAN]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000068893ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxCompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeSodium [(2,3-dihydro-1,5-dimethyl-3-oxo-2-phenyl-1H-pyrazol-4-yl)methylamino]methanesulphonatehttps://echa.europa.eu/substance-information/-/substanceinfo/100.000.631Sodium [(2,3-dihydro-1,5-dimethyl-3-oxo-2-phenyl-1H-pyrazol-4-yl)methylamino]methanesulphonate (EC: 200-694-7)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/118030
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingMETAMIZOLE SODIUMhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/VSU62Z74ON
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/
- ChEBIMetamizole sodiumhttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:59033
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jsp
- Drug Gene Interaction database (DGIdb)LICENSEThe data used in DGIdb is all open access and where possible made available as raw data dumps in the downloads section.http://www.dgidb.org/downloadsMETAMIZOLE SODIUMhttps://www.dgidb.org/drugs/rxcui:1537943
- DailyMed
- Drugs and Lactation Database (LactMed)
- EU Clinical Trials Register
- FDA Approved Animal Drug Products (Green Book)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/MetamizoleNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlAnatomical Therapeutic Chemical (ATC) classificationhttp://www.genome.jp/kegg-bin/get_htext?br08303.keg
- MassBank Europe
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-law
- SpectraBaseMethampyronehttps://spectrabase.com/spectrum/850vYSvNu6DMethampyronehttps://spectrabase.com/spectrum/GwQcwP93SnH(antipyrinylmethylamino)methanesulfonic acid, sodium salthttps://spectrabase.com/spectrum/6ZgtL5nigu2
- Metabolomics Workbench
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.htmlmetamizole sodiumhttps://rxnav.nlm.nih.gov/id/rxnorm/1537943
- WHO Anatomical Therapeutic Chemical (ATC) ClassificationLICENSEUse of all or parts of the material requires reference to the WHO Collaborating Centre for Drug Statistics Methodology. Copying and distribution for commercial purposes is not allowed. Changing or manipulating the material is not allowed.https://www.whocc.no/copyright_disclaimer/Metamizole sodiumhttps://www.whocc.no/atc_ddd_index/?code=N02BB02
- PharmGKBLICENSEPharmGKB data are subject to the Creative Commons Attribution-ShareALike 4.0 license (https://creativecommons.org/licenses/by-sa/4.0/).https://www.pharmgkb.org/page/policies
- Springer Nature
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidatametamizole sodiumhttps://www.wikidata.org/wiki/Q422761
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlAntipyreticshttps://www.ncbi.nlm.nih.gov/mesh/68058633Anti-Inflammatory Agents, Non-Steroidalhttps://www.ncbi.nlm.nih.gov/mesh/68000894
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 388353300https://pubchem.ncbi.nlm.nih.gov/substance/388353300
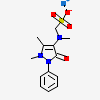

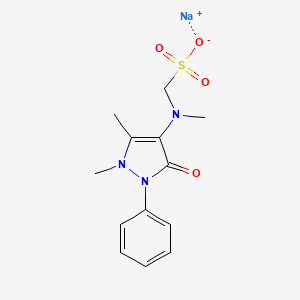
 CID 3111 (Metamizole)
CID 3111 (Metamizole) CID 5360545 (Sodium)
CID 5360545 (Sodium)


