Anavar
- 53-39-4
- Anavar
- 17-epi-Oxandrolone
- 1-hydroxy-1,9a,11a-trimethyl-2,3,3a,3b,4,5,5a,6,9,9b,10,11-dodecahydroindeno[4,5-h]isochromen-7-one
- 17-.beta.-hydroxy-17-methyl-2-oxa-androstan-3-one
- Create:2005-03-26
- Modify:2025-02-01
 Oxandrolone (annotation moved to).
Oxandrolone (annotation moved to).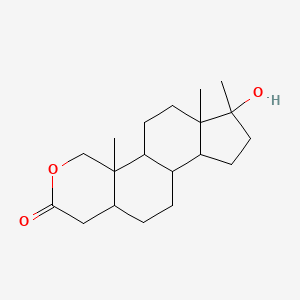
- Anavar
- Oxandrin
- Oxandrolone
- SC 11585
- SC-11585
- SC11585
- 53-39-4
- Anavar
- 17-epi-Oxandrolone
- 1-hydroxy-1,9a,11a-trimethyl-2,3,3a,3b,4,5,5a,6,9,9b,10,11-dodecahydroindeno[4,5-h]isochromen-7-one
- 17-.beta.-hydroxy-17-methyl-2-oxa-androstan-3-one
- 17.beta.-Hydroxy-17-methyl-2-oxa-5.alpha.-androstan-3-one
- 2-Oxa-5.alpha.-androstan-3-one, 17.beta.-hydroxy-17-methyl-
- NSC67068
- 8075CB
- SCHEMBL344040
- 17.beta.-Hydroxy-17.alpha.-methyl-2-oxa-5.alpha.-androstan-3-one
- 2-Oxaandrostan-3-one, 17-hydroxy-17-methyl-, (5.alpha.,17.beta.)-
- Oxandrin; SC-11585; Anavar
- QSLJIVKCVHQPLV-UHFFFAOYSA-N
- 1H-Benz(e)indene-7-acetic acid, dodecahydro-3-hydroxy-6-(hydroxymethyl)-3,3a,6-trimethyl-, .delta.-lactone
- BCP30940
- DB-052328
- NS00014351
- 2-Oxaandrostan-3-one, (5.alpha.,17.beta.)-
- A829561
- 1H-Benz[e]indene-7-acetic acid,3a,6-trimethyl-, .delta.-lactone
- 1,9a,11a-trimethyl-1-oxidanyl-2,3,3a,3b,4,5,5a,6,9,9b,10,11-dodecahydroindeno[4,5-h]isochromen-7-one
- 1-hydroxy-1,9a,11a-trimethyl-2,3,3a,3b,4,5,5a,6,9,9b,10,11-dodecahydroindeno[4,5-h][2]benzopyran-7-one
 Oxandrolone (annotation moved to)
Oxandrolone (annotation moved to)Use (kg) in USA (2002): 29
Consumption (g per capita) in the USA (2002): 0.000101
Calculated removal (%): 49.5


H312 (96.6%): Harmful in contact with skin [Warning Acute toxicity, dermal]
H332 (96.6%): Harmful if inhaled [Warning Acute toxicity, inhalation]
H351 (30.5%): Suspected of causing cancer [Warning Carcinogenicity]
H360 (94.9%): May damage fertility or the unborn child [Danger Reproductive toxicity]
P203, P261, P271, P280, P302+P352, P304+P340, P317, P318, P321, P362+P364, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 59 reports by companies from 5 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Reported as not meeting GHS hazard criteria per 1 of 59 reports by companies. For more detailed information, please visit ECHA C&L website.
There are 4 notifications provided by 58 of 59 reports by companies with hazard statement code(s).
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Acute Tox. 4 (96.6%)
Acute Tox. 4 (96.6%)
Carc. 2 (30.5%)
Repr. 1B (94.9%)
- DTP/NCILICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuse
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeOxandrolone (EC: 200-172-9)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/68171
- Hazardous Substances Data Bank (HSDB)
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/OXANDROLONENORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawOxandrolonehttp://www.nist.gov/srd/nist1a.cfm
- SpectraBaseOxandrolonehttps://spectrabase.com/spectrum/53a5J92MXYy
- Wikidata
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlOxandrolonehttps://www.ncbi.nlm.nih.gov/mesh/68010074Anabolic Agentshttps://www.ncbi.nlm.nih.gov/mesh/68045930
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- NCBI
