Sodium Demethylcantharidate
- Endothal-disodium
- 13114-29-9
- Sodium Demethylcantharidate
- 129-67-9
- Sodium 7-oxabicyclo[2.2.1]heptane-2,3-dicarboxylate
- Create:2005-08-08
- Modify:2025-01-18

- Cotazym S
- Cotazym-S
- Cotazyme
- Creon
- Encron
- Ilozyme
- Ku Zyme
- Ku-Zyme
- Lipram
- Pancrease
- Pancrecarb
- Pancrelipase
- Pancron
- Panokase
- Pertzye
- Protilase
- Ultrase
- Viokase
- Zymase
- Endothal-disodium
- 13114-29-9
- Sodium Demethylcantharidate
- 129-67-9
- Sodium 7-oxabicyclo[2.2.1]heptane-2,3-dicarboxylate
- disodium;7-oxabicyclo[2.2.1]heptane-2,3-dicarboxylate
- 53608-75-6
- Sodium norcantharidin
- Disodium endothal
- DEMETHYLCANTHARIDATE DISODIUM
- ENDOTHALL-SODIUM
- Sodium-Demethylcantharidate
- SCHEMBL1006891
- DTXSID1041899
- CHEBI:81916
- XRHVZWWRFMCBAZ-UHFFFAOYSA-L
- AKOS030633011
- NS00079305
- C18724
- Q27155657
- Sodium3-carboxy-7-oxabicyclo[2.2.1]heptane-2-carboxylate
- Pancrelipase (annotation moved to)
Endothal-disodium (annotation moved to)

H301: Toxic if swallowed [Danger Acute toxicity, oral]
H312: Harmful in contact with skin [Warning Acute toxicity, dermal]
H315: Causes skin irritation [Warning Skin corrosion/irritation]
H319: Causes serious eye irritation [Warning Serious eye damage/eye irritation]
H335: May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation]
P261, P264, P264+P265, P270, P271, P280, P301+P316, P302+P352, P304+P340, P305+P351+P338, P317, P319, P321, P330, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Acute Tox. 3 *
Acute Tox. 4 *
STOT SE 3
Skin Irrit. 2
Eye Irrit. 2
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=XRHVZWWRFMCBAZ-UHFFFAOYSA-L
- ChEBIEndothal-disodiumhttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:81916
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- EPA Chemical and Products Database (CPDat)Disodium endothalhttps://comptox.epa.gov/dashboard/DTXSID1041899#exposureEPA CPDat Classificationhttps://www.epa.gov/chemical-research/chemical-and-products-database-cpdat
- EPA DSSToxDisodium endothalhttps://comptox.epa.gov/dashboard/DTXSID1041899CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/
- EPA Pesticide Ecotoxicity Database
- EU Clinical Trials Register
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingPancrelipasehttp://www.hmdb.ca/metabolites/HMDB0304827
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.html
- Regulation (EC) No 1272/2008 of the European Parliament and of the CouncilLICENSEThe copyright for the editorial content of this source, the summaries of EU legislation and the consolidated texts, which is owned by the EU, is licensed under the Creative Commons Attribution 4.0 International licence.https://eur-lex.europa.eu/content/legal-notice/legal-notice.htmlendothal-sodium (ISO); disodium 7-oxabicyclo(2,2,1)heptane-2,3-dicarboxylatehttps://eur-lex.europa.eu/eli/reg/2008/1272/oj
- Springer Nature
- USGS Columbia Environmental Research CenterLICENSEhttps://www.usgs.gov/foiaENDOTHALL DES-I-CATEhttps://www.cerc.usgs.gov/data/acute/qrychemdesc.asp?Chemical=E0060
- WikidataEndothal-disodiumhttps://www.wikidata.org/wiki/Q27155657
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlPancrelipasehttps://www.ncbi.nlm.nih.gov/mesh/68020799Gastrointestinal Agentshttps://www.ncbi.nlm.nih.gov/mesh/68005765
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- EPA Chemicals under the TSCAEPA TSCA Classificationhttps://www.epa.gov/tsca-inventory
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 389601069https://pubchem.ncbi.nlm.nih.gov/substance/389601069
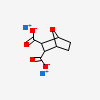

 CID 3225 (Endothall)
CID 3225 (Endothall) CID 5360545 (Sodium)
CID 5360545 (Sodium)