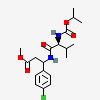
Valifenalate
- Valifenalate
- 283159-90-0
- Valiphenal
- Valifenalate [ISO]
- S4BR27QQ5S
- Create:2006-10-26
- Modify:2025-01-18
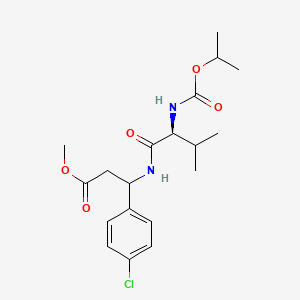

- Valifenalate
- 283159-90-0
- Valiphenal
- Valifenalate [ISO]
- S4BR27QQ5S
- IR 5885
- DTXSID6058051
- CHEBI:83610
- IR5885;Valiphenal
- methyl 3-(4-chlorophenyl)-3-[[(2S)-3-methyl-2-(propan-2-yloxycarbonylamino)butanoyl]amino]propanoate
- IR5885
- methyl 3-(4-chlorophenyl)-3-{[N-(isopropoxycarbonyl)-L-valyl]amino}propanoate
- methyl N-(isopropoxycarbonyl)-L-valyl-3-(4-chlorophenyl)-beta-alaninate
- methyl N-(isopropoxycarbonyl)-L-valyl-3-(p-chlorophenyl)-beta-alaninate
- methyl N-[(1-methylethoxy)carbonyl]-L-valyl-3-(4-chlorophenyl)-beta-alaninate
- beta-Alanine, N-((1-methylethoxy)carbonyl)-L-valyl-3-(4-chlorophenyl)-, methyl ester
- METHYL (3RS)-3-(4-CHLOROPHENYL)-N-(N-(ISOPROPOXYCARBONYL)-L-VALYL)-.BETA.-ALANINATE
- Methyl (3RS)-3-(4-chlorophenyl)-N-(N-(isopropoxycarbonyl)-L-valyl)-beta-alaninate
- METHYL 3-(4-CHLOROPHENYL)-N-(((1-METHYLETHOXY)CARBONYL)-L-VALYL)-.BETA.-ALANINATE
- Methyl 3-(4-chlorophenyl)-N-(((1-methylethoxy)carbonyl)-L-valyl)-beta-alaninate
- .BETA.-ALANINE, N-((1-METHYLETHOXY)CARBONYL)-L-VALYL-3-(4-CHLOROPHENYL)-, METHYL ESTER
- beta-Alanine, N-[(1-methylethoxy)carbonyl]-L-valyl-3-(4-chlorophenyl)-, methyl ester
- methyl 3-(4-chlorophenyl)-3-((N-(isopropoxycarbonyl)-L-valyl)amino)propanoate
- methyl N-((1-methylethoxy)carbonyl)-L-valyl-3-(4-chlorophenyl)-beta-alaninate
- N-((1-methylethoxy)carbonyl)-L-valyl-3-(4-chlorophenyl)-b-Alanine, methyl ester
- N-[(1-methylethoxy)carbonyl]-L-valyl-3-(4-chlorophenyl)-b-Alanine, methyl ester
- Valifenalate isomer 1
- Valifenalate isomer 2
- Valifenalate (Standard)
- UNII-S4BR27QQ5S
- SCHEMBL1138368
- DTXCID8031819
- EX-A3342
- HY-17518R
- CS-3304
- AS-77009
- DA-78818
- HY-17518
- NS00098472
- D94923
- Valifenalate, PESTANAL(R), analytical standard
- Q19310729
- Benzenepropanoic acid, 4-chloro-.beta.-[[(2S)-3-methyl-2-[[(1-methylethoxy)carbonyl]amino]-1-oxobutyl]amino]-, methyl ester
208.43 Ų [M-H]-
208.51 Ų [M+HCOO]-
208.16 Ų [M+HCOO]-
207.08 Ų [M+Cl]-
206.93 Ų [M+Cl]-
208.44 Ų [M-H]-


H351 (98.9%): Suspected of causing cancer [Warning Carcinogenicity]
H411 (100%): Toxic to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard]
P203, P273, P280, P318, P391, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 88 reports by companies from 2 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Carc. 2 (98.9%)
Aquatic Chronic 2 (100%)
Carc. 2
Aquatic Chronic 2
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=DBXFMOWZRXXBRN-LWKPJOBUSA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxValifenalatehttps://comptox.epa.gov/dashboard/DTXSID6058051CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- EPA Safe Drinking Water Act (SDWA)
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticemethyl N-(isopropoxycarbonyl)-L-valyl-(3RS)-3-(4-chlorophenyl)-β-alaninate; valifenalatehttps://echa.europa.eu/substance-information/-/substanceinfo/100.125.911methyl N-(isopropoxycarbonyl)-L-valyl-(3RS)-3-(4-chlorophenyl)-β-alaninate; valifenalate (EC: 608-192-3)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/97947
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Risk Assessment Information System (RAIS)LICENSEThis work has been sponsored by the U.S. Department of Energy (DOE), Office of Environmental Management, Oak Ridge Operations (ORO) Office through a joint collaboration between United Cleanup Oak Ridge LLC (UCOR), Oak Ridge National Laboratory (ORNL), and The University of Tennessee, Ecology and Evolutionary Biology, The Institute for Environmental Modeling (TIEM). All rights reserved.https://rais.ornl.gov/Valifenalatehttps://rais.ornl.gov/cgi-bin/tools/TOX_search
- ChEBI
- EPA Pesticide Ecotoxicity Database
- EU Pesticides DatabaseValifenalate (formerly Valiphenal)https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/start/screen/active-substances/details/189
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/ValifenalateNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- USDA Pesticide Data ProgramValifenalatehttps://www.ams.usda.gov/datasets/pdp
- Regulation (EC) No 1272/2008 of the European Parliament and of the CouncilLICENSEThe copyright for the editorial content of this source, the summaries of EU legislation and the consolidated texts, which is owned by the EU, is licensed under the Creative Commons Attribution 4.0 International licence.https://eur-lex.europa.eu/content/legal-notice/legal-notice.htmlmethyl N-(isopropoxycarbonyl)-L-valyl-(3RS)-3-(4-chlorophenyl)-β-alaninate;...https://eur-lex.europa.eu/eli/reg/2008/1272/oj
- Japan Chemical Substance Dictionary (Nikkaji)
- Springer Nature
- Wikidatavalifenalatehttps://www.wikidata.org/wiki/Q19310729
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 389637423https://pubchem.ncbi.nlm.nih.gov/substance/389637423