Tafamidis Meglumine
- Tafamidis meglumine
- 951395-08-7
- Vyndaqel
- Fx-1006A
- Fx1006A
- Create:2008-10-20
- Modify:2025-01-18
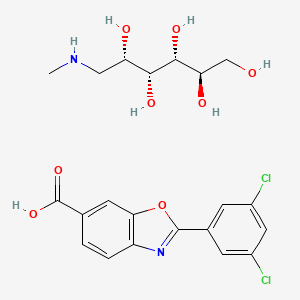
- FX 1006A
- FX-1006A
- FX1006A
- tafamidis
- tafamidis meglumine
- Vyndamax
- Vyndaqel
- Tafamidis meglumine
- 951395-08-7
- Vyndaqel
- Fx-1006A
- Fx1006A
- Fx 1006A
- UNII-ZU7CF08A1A
- ZU7CF08A1A
- CHEBI:79345
- (2R,3R,4R,5S)-6-(Methylamino)hexane-1,2,3,4,5-pentaol 2-(3,5-dichlorophenyl)benzo[d]oxazole-6-carboxylate
- 951395-08-7 (meglumine)
- D-Glucitol, 1-deoxy-1-(methylamino)-, 2-(3,5-dichlorophenyl)-6-benzoxazolecarboxylate
- 1-deoxy-1-(methylazaniumyl)-D-glucitol 2-(3,5-dichlorophenyl)-1,3-benzoxazole-6-carboxylate
- (2R,3R,4R,5S)-6-(methylamino)hexane-1,2,3,4,5-pentaol 2-(3,5-dichlorophenyl)benzo[d]oxazole-6-carboxylic acid
- 2-(3,5-dichlorophenyl)-1,3-benzoxazole-6-carboxylic acid--1-deoxy-1-(methylamino)-D-glucitol (1/1)
- 2-(3,5-dichlorophenyl)-1,3-benzoxazole-6-carboxylic acid;(2R,3R,4R,5S)-6-(methylamino)hexane-1,2,3,4,5-pentol
- D-GLUCITOL, 1-DEOXY-1-(METHYLAMINO)-, 2-(3,5-DICHLOROPHENYL)-6-BENZOXAZOLECARBOXYLATE (1:1)
- D-GLUCO-2,3,4,5,6-PENTAHYDROXY-N-METHYLHEXAN-1-AMINIUM 2-(3,5-DICHLOROPHENYL)BENZOXAZOLE-6-CARBOXYLATE
- Tafamidis meglumine [USAN]
- Tafamidis meglumine [USAN:INN]
- Vyndaqel (TN)
- Tafamidis meglumine?
- C21H24Cl2N2O8
- CHEMBL2105675
- SCHEMBL14783506
- TAFAMIDIS MEGLUMINE [MI]
- Tafamidis meglumine (JAN/USAN)
- DTXSID50915094
- TAFAMIDIS MEGLUMINE [JAN]
- EX-A3550
- HY-14852A
- MFCD28502032
- TAFAMIDIS MEGLUMINE [WHO-DD]
- AKOS037649321
- AT18247
- TAFAMIDIS MEGLUMINE [ORANGE BOOK]
- BS-18043
- DA-78162
- CS-0045567
- PF-06291826
- D09674
- Q27148396
- 2-(3,5-Dichlorophenyl)-1,3-benzoxazole-6-carboxylic acid mono(1-deoxy-1-methylamino-D-glucitol)
- 2-(3,5-Dichlorophenyl)-1,3-benzoxazole-6-carboxylic acid--1-deoxy-1-(methylamino)hexitol (1/1)
 Tafamidis (has active moiety)
Tafamidis (has active moiety)

H302 (20%): Harmful if swallowed [Warning Acute toxicity, oral]
H312 (20%): Harmful in contact with skin [Warning Acute toxicity, dermal]
H315 (20%): Causes skin irritation [Warning Skin corrosion/irritation]
H319 (20%): Causes serious eye irritation [Warning Serious eye damage/eye irritation]
H332 (20%): Harmful if inhaled [Warning Acute toxicity, inhalation]
H360 (80%): May damage fertility or the unborn child [Danger Reproductive toxicity]
H360D (40%): May damage the unborn child [Danger Reproductive toxicity]
H362 (20%): May cause harm to breast-fed children [Reproductive toxicity, effects on or via lactation]
P203, P260, P261, P263, P264, P264+P265, P270, P271, P280, P301+P317, P302+P352, P304+P340, P305+P351+P338, P317, P318, P321, P330, P332+P317, P337+P317, P362+P364, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 5 reports by companies from 4 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Acute Tox. 4 (20%)
Acute Tox. 4 (20%)
Skin Irrit. 2 (20%)
Eye Irrit. 2 (20%)
Acute Tox. 4 (20%)
Repr. 1A (80%)
Lact. (20%)
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=DQJDBUPLRMRBAB-WZTVWXICSA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/Tafamidis megluminehttps://commonchemistry.cas.org/detail?cas_rn=951395-08-7
- ChemIDplusTafamidis meglumine [USAN:INN]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0951395087ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSTox2-(3,5-Dichlorophenyl)-1,3-benzoxazole-6-carboxylic acid--1-deoxy-1-(methylamino)hexitol (1/1)https://comptox.epa.gov/dashboard/DTXSID50915094CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-notice2-(3,5-dichlorophenyl)-1,3-benzoxazole-6-carboxylic acid;(2R,3R,4R,5S)-6-(methylamino)hexane-1,2,3,4,5-pentolhttps://echa.europa.eu/substance-information/-/substanceinfo/100.246.0202-(3,5-dichlorophenyl)-1,3-benzoxazole-6-carboxylic acid;(2R,3R,4R,5S)-6-(methylamino)hexane-1,2,3,4,5-pentol (EC: 813-716-0)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/253405
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingTAFAMIDIS MEGLUMINEhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/ZU7CF08A1A
- ChEBITafamidis megluminehttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:79345
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_use
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licenceTAFAMIDIS MEGLUMINEhttps://platform.opentargets.org/drug/CHEMBL2105675
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jsp
- Drug Gene Interaction database (DGIdb)LICENSEThe data used in DGIdb is all open access and where possible made available as raw data dumps in the downloads section.http://www.dgidb.org/downloadsTAFAMIDIS MEGLUMINEhttps://www.dgidb.org/drugs/rxcui:1727003
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- DailyMed
- European Medicines Agency (EMA)LICENSEInformation on the European Medicines Agency's (EMA) website is subject to a disclaimer and copyright and limited reproduction notices.https://www.ema.europa.eu/en/about-us/legal-noticeTafamidis meglumine (P/147/2010)https://www.ema.europa.eu/en/medicines/human/paediatric-investigation-plans/emea-000884-pip01-10
- Drugs@FDALICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingTAFAMIDIS MEGLUMINEhttps://www.accessdata.fda.gov/scripts/cder/daf/
- EU Clinical Trials Register
- FDA Orange BookLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Japan Pharmaceuticals and Medical Devices Agency (PMDA)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlTherapeutic category of drugs in Japanhttp://www.genome.jp/kegg-bin/get_htext?br08301.kegUSP drug classificationhttp://www.genome.jp/kegg-bin/get_htext?br08302.kegAnatomical Therapeutic Chemical (ATC) classificationhttp://www.genome.jp/kegg-bin/get_htext?br08303.kegTarget-based classification of drugshttp://www.genome.jp/kegg-bin/get_htext?br08310.keg
- National Drug Code (NDC) DirectoryLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- NIPH Clinical Trials Search of Japan
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.htmltafamidis megluminehttps://rxnav.nlm.nih.gov/id/rxnorm/1727003
- Therapeutic Target Database (TTD)Tafamidis megluminehttps://idrblab.net/ttd/data/drug/details/D03MGL
- Springer Nature
- Wikidatatafamidis megluminehttps://www.wikidata.org/wiki/Q27148396
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.html
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 392273231https://pubchem.ncbi.nlm.nih.gov/substance/392273231
 CID 8567 (Meglumine)
CID 8567 (Meglumine)