Sulfacetamide Sodium
- 6209-17-2
- Sulfacetamide sodium monohydrate
- Sulfacetamide sodium salt hydrate
- Sodium sulfacetamide
- Klaron
- Create:2006-04-14
- Modify:2025-02-01
 Sulfacetamide (has active moiety); Prednisolone acetate; sulfacetamide sodium (component of); Sulfacetamide Sodium; Sulfur (component of) ... View More ...
Sulfacetamide (has active moiety); Prednisolone acetate; sulfacetamide sodium (component of); Sulfacetamide Sodium; Sulfur (component of) ... View More ...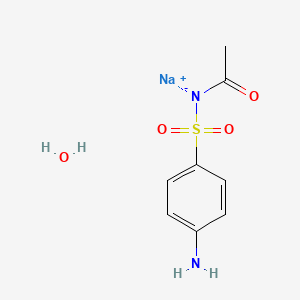
- Brandiazin
- Dermazin
- Flamazine
- Flammazine
- Sicazine
- Silvadene
- Silvederma
- Silver Sulfadiazine
- Silver Sulfafdiazine
- SSD
- SSD AF
- Sulfadiazine, Silver
- Sulfafdiazine, Silver
- Sulfargen
- Thermazene
- 6209-17-2
- Sulfacetamide sodium monohydrate
- Sulfacetamide sodium salt hydrate
- Sodium sulfacetamide
- Klaron
- Flammazine
- Ocusulf
- Sulfacel
- Sulfair Forte
- Sulfacetamide sodium salt monohydrate
- Ocusulf-10
- Sulfair 10
- Sulfair-15
- Sulten-10
- Sodium sulamyd
- Sulf-15
- Bleph
- Sulfacel-15
- Sulamyd sodium monohydrate
- OCUSULF-30
- Sulfacetamide sodic hydrate
- Sulphacetamide sodium
- UNII-4NRT660KJQ
- sulfacetamide sodium hydrate
- BLEPH-30
- Sulfacetamide sodium [USP]
- 4NRT660KJQ
- Isopto cetamide
- Sodium N-sulfanilylacetamide monohydrate
- Sulfacetamide sodium salt
- CHEBI:63858
- sodium;acetyl-(4-aminophenyl)sulfonylazanide;hydrate
- Sulfacetamidum natricum
- MFCD00149555
- NSC-625324
- Sulfacetamide sodium (USP)
- N1-Acetylsulfanilamide sodium monohydrate
- Sulfacetamide (sodium monohydrate)
- Sodium sulfacetamide, anhydrous
- DTXSID50211129
- N-Sulfanilylacetamide monosodium salt monohydrate
- Almocetamide
- Sulfableph
- Albucide
- Blefamide
- Farmamid
- Galseptil
- Octsetan
- Prontamid
- Sodium acetyl((4-aminophenyl)sulfonyl)amide hydrate
- Locula
- Albucid soluble
- Sodium sulfacyl
- Sulfacyl sodium
- Beocid-isoptal
- Sebizon lotion
- Sodium albucid
- Sulamyd sodium
- Sulfacyl sol
- Acetamide, N-sulfanilyl-, monosodium salt, monohydrate
- Soluble sulfacyl
- Sulfacyl soluble
- FML-S COMPONENT SULFACETAMIDE SODIUM
- Sulfacetamid natrium
- METIMYD COMPONENT SULFACETAMIDE SODIUM
- SULSTER COMPONENT SULFACETAMIDE SODIUM
- Acetamide, N-((4-aminophenyl)sulfonyl)-, monosodium salt, monohydrate
- Minims sulphacetamide
- Soluble sulfacetamide
- Sulfacyl sodium salt
- CETAPRED COMPONENT SULFACETAMIDE SODIUM
- MFCD00007885
- SULPHRIN COMPONENT SULFACETAMIDE SODIUM
- Oc-U-Mid
- Sulf-10 Ophthalmic
- PREDAMIDE COMPONENT SULFACETAMIDE SODIUM
- Sulfacetamide, sodium
- Opulets sulpjacetamide
- Bleph 10
- BLEPHAMIDE COMPONENT SULFACETAMIDE SODIUM
- PREDSULFAIR COMPONENT SULFACETAMIDE SODIUM
- ISOPTO CETAPRED COMPONENT SULFACETAMIDE SODIUM
- BLEPHAMIDE S.O.P. COMPONENT SULFACETAMIDE SODIUM
- Sulfacetamide, sodium salt
- SULFACETAMIDE SODIUM (II)
- SULFACETAMIDE SODIUM [II]
- N-Sulfanilylacetamide sodium
- SULFACETAMIDE SODIUM (MART.)
- SULFACETAMIDE SODIUM [MART.]
- SULFACETAMIDE SODIUM (USP-RS)
- SULFACETAMIDE SODIUM [USP-RS]
- N(1)-acetylsulfanilamide sodium
- SULFACETAMIDE SODIUM (EP IMPURITY)
- SULFACETAMIDE SODIUM [EP IMPURITY]
- SULFACETAMIDE SODIUM (EP MONOGRAPH)
- SULFACETAMIDE SODIUM [EP MONOGRAPH]
- UNII-30760ZE777
- SULFACETAMIDE SODIUM (USP MONOGRAPH)
- SULFACETAMIDE SODIUM [USP MONOGRAPH]
- NSC63871
- EINECS 204-848-4
- N(sup 1)-Acetylsulfanilamide sodium
- N(1)-acetylsulfanilamide sodium salt
- Acetamide, N-sulfanilyl-, sodium deriv
- Sodium, N(sup 1)-acetylsulfanilamido-
- Acetamide, N-sulfanilyl-, N-sodium deriv
- N(sup 1)-Acetylsulfanilamide sodium salt
- Sodium Acetyl[(4-aminophenyl)sulfonyl]amide Hydrate
- N-SULFANILYLACETAMIDE MONOSODIUM SALT
- sodium acetyl((4-aminophenyl)sulfonyl)azanide
- sodium acetyl((4-aminophenyl)sulfonyl)azanide--water (1/1)
- sodium acetyl[(4-aminophenyl)sulfonyl]azanide--water (1/1)
- Plexion
- Prascion
- Rosaderm
- Sulfatol
- Sumaxin
- Topisulf
- Cerisa
- Claris
- Rosula
- Ovace
- OvacePlus
- OvacePlusPlus
- Zencia wash
- ACETAMIDE, N-SULFANILYL-, MONOSODIUM SALT
- Ovace Plus
- Cetamide (TN)
- Prestwick_427
- Klaron (TN)
- Bleph (TN)
- PLEXION NS
- Sodium SulfacetamideWash
- Sulfacetamidesodiummonohydrate
- MLS002154172
- Sodium Sulfacetamide Wash 10%
- CHEMBL1723241
- DTXCID00133620
- CNTXYOLKLFYMSC-UHFFFAOYSA-M
- HMS1568C09
- HMS2095C09
- HMS2231L18
- HMS3372D13
- HMS3712C09
- N-[(4-aminophenyl)sulfonyl]acetamide, oxamethane, sodium salt
- HY-B0888
- SULFACETAMIDE SODIUM [VANDF]
- s4750
- STK023979
- SULFACETAMIDE SODIUM [WHO-IP]
- AKOS000489082
- AKOS005379705
- S4750 Sulfacetamide sodium salt hydr
- CCG-220014
- Exact-Rx Sodium Sulfacetamide Wash 10%
- NCGC00017061-01
- NCGC00017061-02
- AS-81746
- SMR001233470
- SULFACETAMIDE SODIUM [ORANGE BOOK]
- SY076524
- CAS-6209-17-2
- SULFACETAMIDUM NATRICUM [WHO-IP LATIN]
- D00871
- G63588
- SULFACETAMIDE SODIUM COMPONENT OF FML-S
- sodium acetyl(4-aminophenylsulfonyl)amide hydrate
- SULFACETAMIDE SODIUM COMPONENT OF CETAPRED
- SULFACETAMIDE SODIUM COMPONENT OF METIMYD
- SULFACETAMIDE SODIUM COMPONENT OF SULPHRIN
- SULFACETAMIDE SODIUM COMPONENT OF SULSTER
- SR-01000842154
- SULFACETAMIDE SODIUM COMPONENT OF BLEPHAMIDE
- SULFACETAMIDE SODIUM COMPONENT OF PREDAMIDE
- SULFACETAMIDE SODIUM SALT MONOHYDRATE [MI]
- SR-01000842154-2
- SULFACETAMIDE SODIUM COMPONENT OF PREDSULFAIR
- Q27132864
- sodium acetyl[(4-aminophenyl)sulfonyl]azanide hydrate
- SULFACETAMIDE SODIUM COMPONENT OF ISOPTO CETAPRED
- SULFACETAMIDE SODIUM COMPONENT OF BLEPHAMIDE S.O.P.
- Sulfacetamide sodium salt monohydrate, >=98.0% (dried material, T)
- Sulfacetamide sodium, United States Pharmacopeia (USP) Reference Standard
Sulfacetamide (has active moiety)
- Prednisolone acetate; sulfacetamide sodium (component of)
- Prednisolone sodium phosphate; sulfacetamide sodium (component of)
- Sulfacetamide Sodium; Sulfur (component of)
- Fluorometholone; sulfacetamide sodium (component of)
- Salicylic acid; sulfacetamide sodium (component of)
- Niacinamide; sulfacetamide sodium (component of)
- Clindamycin phosphate; sulfacetamide sodium; tretinoin (component of)

H315 (93%): Causes skin irritation [Warning Skin corrosion/irritation]
H319 (93%): Causes serious eye irritation [Warning Serious eye damage/eye irritation]
H335 (93%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation]
P261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 43 reports by companies from 5 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Reported as not meeting GHS hazard criteria per 2 of 43 reports by companies. For more detailed information, please visit ECHA C&L website.
There are 4 notifications provided by 41 of 43 reports by companies with hazard statement code(s).
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Skin Irrit. 2 (93%)
Eye Irrit. 2 (93%)
STOT SE 3 (93%)
Skin Sens. 1 (16.7%)
Resp. Sens. 1 (16.7%)
IMAP assessments - Acetamide, N-[(4-aminophenyl)sulfonyl]-, monosodium salt, monohydrate: Environment tier I assessment
IMAP assessments - Acetamide, N-[(4-aminophenyl)sulfonyl]-, monosodium salt, monohydrate: Human health tier I assessment
IMAP assessments - Acetamide, N-[(4-aminophenyl)sulfonyl]-, monosodium salt: Environment tier I assessment
IMAP assessments - Acetamide, N-[(4-aminophenyl)sulfonyl]-, monosodium salt: Human health tier I assessment
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=IHCDKJZZFOUARO-UHFFFAOYSA-M
- Australian Industrial Chemicals Introduction Scheme (AICIS)Acetamide, N-[(4-aminophenyl)sulfonyl]-, monosodium salt, monohydratehttps://services.industrialchemicals.gov.au/search-assessments/
- ChemIDplusSulfacetamide sodium [USP]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0006209172ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA Chemicals under the TSCAAcetamide, N-[(4-aminophenyl)sulfonyl]-, sodium salt (1:1)https://www.epa.gov/chemicals-under-tsca
- EPA DSSToxSulfacetamide sodium [USP]https://comptox.epa.gov/dashboard/DTXSID50211129CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeSulfacetamide sodiumhttps://echa.europa.eu/substance-information/-/substanceinfo/100.004.409Sulfacetamide sodiumhttps://echa.europa.eu/substance-information/-/substanceinfo/100.170.810Sulfacetamide sodium (EC: 204-848-4)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/83256Sulfacetamide sodium (EC: 642-939-4)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/175705
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingSULFACETAMIDE SODIUMhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/4NRT660KJQ
- ChEBISulfacetamide sodiumhttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:63858
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licenceSULFACETAMIDE SODIUMhttps://platform.opentargets.org/drug/CHEMBL1723241
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.html
- DailyMed
- Drugs@FDALICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingSULFACETAMIDE SODIUMhttps://www.accessdata.fda.gov/scripts/cder/daf/
- EU Clinical Trials Register
- FDA Orange BookLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlUSP drug classificationhttp://www.genome.jp/kegg-bin/get_htext?br08302.kegAnatomical Therapeutic Chemical (ATC) classificationhttp://www.genome.jp/kegg-bin/get_htext?br08303.kegAntiinfectiveshttp://www.genome.jp/kegg-bin/get_htext?br08307.keg
- National Drug Code (NDC) DirectoryLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.htmlsulfacetamide sodiumhttps://rxnav.nlm.nih.gov/id/rxnorm/82101
- Wikidatasulfacetamide sodium hydratehttps://www.wikidata.org/wiki/Q27132864
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlSilver Sulfadiazinehttps://www.ncbi.nlm.nih.gov/mesh/68012837Anti-Infective Agents, Localhttps://www.ncbi.nlm.nih.gov/mesh/68000891
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 388010780https://pubchem.ncbi.nlm.nih.gov/substance/388010780


 CID 5360545 (Sodium)
CID 5360545 (Sodium) CID 962 (Water)
CID 962 (Water)