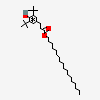
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate
- 2082-79-3
- Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate
- Irganox 1076
- Antioxidant 1076
- Tominokusu SS
- Create:2005-03-28
- Modify:2025-01-18
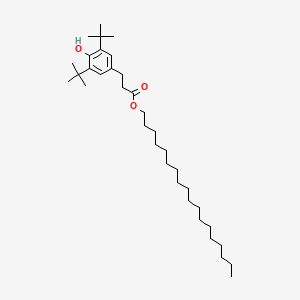
- Irganox 1076
- n-octadecyl beta-(3',5'-di-tert-butyl-4'-hydroxyphenyl)propionate
- stearyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate
- 2082-79-3
- Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate
- Irganox 1076
- Antioxidant 1076
- Tominokusu SS
- octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propanoate
- Naugard 76
- Ralox 530
- Sumilizer BP 76
- Ultranox 276
- stearyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate
- Irganox 1906
- Irganox 1976
- Irganox L 107
- Irganox I 1076
- ADK Stab AO 50
- Anox PP 18
- Mark AO 50
- Octadecyl 3-(3,5-ditert-butyl-4-hydroxyphenyl)propanoate
- Stearyl 3,5-di-tert-butyl-4-hydroxyhydrocinnamate
- IR 1076
- Octadecyl 3,5-di-tert-butyl-4-hydroxyhydrocinnamate
- Benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, octadecyl ester
- E 376
- U 276
- I 1076
- Octadecyl DI-tert-butyl-4-hydroxyhydrocinnamate
- Hydrocinnamic acid, 3,5-di-tert-butyl-4-hydroxy-, octadecyl ester
- V88J661G2P
- Octadecyl 3-(3',5'-di-tert-butyl-4'-hydroxyphenyl)propionate
- octadecyl di-t-butyl-4-hydroxyhydrocinnamate
- 3,5-Bis(1,1-dimethylethyl)-4-hydroxybenzenepropanoic acid octadecyl ester
- Benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-,octadecyl ester
- Octadecyl 3,5-di-t-butyl-4-hydroxyhydrocinnamate
- Octadecyl 3,5-bis(tert-butyl)-4-hydroxyhydrocinnamate
- C35H62O3
- Octadecyl 3-?(3,?5-?Di-?tert-?butyl-?4-?hydroxyphenyl)?propionate
- HSDB 5865
- EINECS 218-216-0
- Lowinox PO35
- MFCD00017498
- TINOGARD TS
- octadecyl3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate
- PLASTIC ADDITIVE 4
- Dibutylhydroxyphenylpropionic acid stearyl ester
- SELOSOL F 773
- IRGANOX-1076
- SCHEMBL18856
- SONGNOX 1076
- Irganox 1076, 98%
- BIDD:ER0412
- UNII-V88J661G2P
- DTXSID8027456
- SSDSCDGVMJFTEQ-UHFFFAOYSA-
- Stearyl beta-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate
- Hydrocinnamic acid, 3,5-di-t-butyl-4-hydroxy-, octadecyl ester
- n-octadecyl (3-[3,5-di-tert-butyl-4-hydroxyphenyl]propionate)
- PLASTIC ADDITIVE 4 [USP-RS]
- Stearyl 3-(4-hydroxy-3,5-di-tert-butyl-4-hydroxyphenyl)propionate
- AKOS015890027
- 1ST3118
- AC-15275
- AS-12172
- CS-0152197
- D1644
- NS00003190
- Irganox 1076 100 microg/mL in Acetonitrile
- 1ST3118-1000
- D70534
- EC 218-216-0
- octadecyl 3,5-di-t-butyl-4-hydroxy-hydrocinnamate
- octadecyl-3,5-di-t-butyl-4-hydroxyhydrocinnamate
- Antioxidant 1076 Solution in Hexane, 1000mug/mL
- Octadecyl-3,5-di-tert-butyl-4-hydroxyhydrocinnamate
- W-107592
- 2,6-di-tert-butyl-4-(octadecanoxycarbonylethyl)phenol
- n-octadecyl 3,5-di-tert-butyl-4-hydroxyhydrocinnamate
- octadecyl-3,5-di-tert-butyl-4-hydroxy-hydrocinnamate
- Q27291662
- stearyl 3-(4-hydroxy-3,5-di-t-butylphenyl)propionate
- octadecyl 3-(3,5-di-t-butyl-4-hydroxyphenyl)propionate
- STEARYL 3,5-DI-TERT-BUTYL-4-HYDROXHYDROCINNAMATE
- 2,6-Di-tert-butyl-4-[(2-octadecyloxycarbonyl)ethyl]phenol
- OCTADECYL 3,5-DI-TERT-BUTYL-4-OXYPHENYLPROPIONATE
- Octadecyl 3-(3,5-di-t-butyl-4-hydroxyphenyl) propionate
- Octadecyl 3-(3,5-di-tertbutyl-4-hydroxyphenyl)-propionate
- Octadecyl 3-(3,5-ditert-butyl-4-hydroxyphenyl)propanoate #
- Octadecyl-3(3',5'-di-t-butyl-4'-hydroxyphenyl)propionate
- n-octadecyl beta-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate
- n-octadecyl-3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate
- octadecyl-3-(3',5'-di-tert-butyl-4'-hydroxyphenyl)propionate
- Stearyl .beta.-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate
- 3-(3,5-Di-tert-butyl-4-hydroxyphenyl)propionic Acid Stearyl Ester
- n-octadecyl-3-(3',5'-di-tert-butyl-4'-hydroxyphenyl)propionate
- Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate, 99%
- Plastic additive 11, European Pharmacopoeia (EP) Reference Standard
- 3,5-DI-TERT-BUTYL-4-HYDROXYPHENYLPROPIONIC ACID OCTADECYL ESTER
- 3-(3',5'-Di-tert-butyl-4'-hydroxyphenyl)propionic acid stearyl ester
- 3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENOL)PROPIONIC ACID OCTADECYL ESTER
- 3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONIC ACID OCTADECYL ESTER
- beta-(3,5-Di-tert-butyl-4-hydroxyphenyl)propionic acid octadecyl ester
- OCTADECYL .BETA.-(4'-HYDROXY-3',5'-DI-TERT-BUTYLPHENYL)PROPIONATE
- OCTADECYL .BETA.-(4-HYDROXY-3,5-DI-TERT-BUTYLPHENYL)PROPIONATE
- Plastic additive 4, United States Pharmacopeia (USP) Reference Standard
- STEARYL 3-(4-HYDROXY-3,5-DI-TERT-BUTYL-4- HYDROXYPHENYL)PROPIONATE
- HYDROCINNAMIC ACID, 3,5-DI-T-BUTYL-4-HYDROXY-, OCTADECYL ESTER [HSDB]
- OCTADECYL 3-(3,5-BIS(1,1-DIMETHYLETHYL)-4-HYDROXYBENZENE)PROPIONATE
- InChI=1/C35H62O3/c1-8-9-10-11-12-13-14-15-16-17-18-19-20-21-22-23-26-38-32(36)25-24-29-27-30(34(2,3)4)33(37)31(28-29)35(5,6)7/h27-28,37H,8-26H2,1-7H3
57 99.99
43 47.73
530 35.39
515 33.93
55 30.28
57 999
43 477
530 354
515 339
55 303
- Oxidizing/reducing agents
- UV stabilizer
- Antioxidant
- Reducing agent
- Other (specify)
- Defoamer
- Stabilizing agent
- Heat stabilizer
- Not Known or Reasonably Ascertainable
- Diluent
- Paint additives and coating additives not described by other categories
- Processing aids, not otherwise listed
- Chemical reaction regulator
- Processing aids, not otherwise listed
- Not Known or Reasonably Ascertainable
- Antioxidant
- Oxidizing/reducing agents
- UV stabilizer
- Reducing agent
Information on 11 consumer products that contain Hydrocinnamic acid, 3,5-di-t-butyl-4-hydroxy-, octadecyl ester in the following categories is provided:
• Inside the Home
• Personal Care
2019: 20,000,000 lb - <100,000,000 lb
2018: 10,000,000 - <50,000,000 lb
2017: 10,000,000 - <50,000,000 lb
2016: 10,000,000 - <50,000,000 lb
- Not Known or Reasonably Ascertainable
- Plastics Material and Resin Manufacturing
- Synthetic Rubber Manufacturing
- Custom Compounding of Purchased Resins
- All Other Chemical Product and Preparation Manufacturing
- Rubber Product Manufacturing
- Plastics Product Manufacturing
- Other (requires additional information)
- Adhesive Manufacturing
- Paint and Coating Manufacturing
- Machinery Manufacturing
- Transportation Equipment Manufacturing
- Miscellaneous Manufacturing
- Petroleum Lubricating Oil and Grease Manufacturing

P261, P272, P280, P302+P352, P321, P333+P317, P362+P364, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 1592 reports by companies from 14 notifications to the ECHA C&L Inventory.
Reported as not meeting GHS hazard criteria per 1180 of 1592 reports by companies. For more detailed information, please visit ECHA C&L website.
There are 11 notifications provided by 412 of 1592 reports by companies with hazard statement code(s).
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Reproductive toxicity - Category 2
Hazardous to the aquatic environment (Long-term) - Category 2
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=SSDSCDGVMJFTEQ-UHFFFAOYSA-N
- Australian Industrial Chemicals Introduction Scheme (AICIS)Benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, octadecyl esterhttps://services.industrialchemicals.gov.au/search-assessments/Benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, octadecyl esterhttps://services.industrialchemicals.gov.au/search-inventory/
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/Antioxidant 1076https://commonchemistry.cas.org/detail?cas_rn=2082-79-3
- ChemIDplusHydrocinnamic acid, 3,5-di-t-butyl-4-hydroxy-, octadecyl esterhttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0002082793ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA Chemical Data Reporting (CDR)LICENSEThe U.S. Government retains a nonexclusive, royalty-free license to publish or reproduce these documents, or allow others to do so, for U.S. Government purposes. These documents may be freely distributed and used for non-commercial, scientific and educational purposes.https://www.epa.gov/web-policies-and-procedures/epa-disclaimers#copyrightBenzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, octadecyl esterhttps://www.epa.gov/chemical-data-reporting
- EPA Chemicals under the TSCABenzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, octadecyl esterhttps://www.epa.gov/chemicals-under-tscaEPA TSCA Classificationhttps://www.epa.gov/tsca-inventory
- EPA DSSToxOctadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionatehttps://comptox.epa.gov/dashboard/DTXSID8027456CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeOctadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionatehttps://chem.echa.europa.eu/100.016.560Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate (EC: 218-216-0)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/81999
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingOCTADECYL DI-TERT-BUTYL-4-HYDROXYHYDROCINNAMATEhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/V88J661G2P
- Hazardous Substances Data Bank (HSDB)HYDROCINNAMIC ACID, 3,5-DI-T-BUTYL-4-HYDROXY-, OCTADECYL ESTERhttps://pubchem.ncbi.nlm.nih.gov/source/hsdb/5865
- International Fragrance Association (IFRA)LICENSE(c) The International Fragrance Association, 2007-2021. All rights reserved.https://ifrafragrance.org/links/copyrightBenzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, octadecyl esterhttps://ifrafragrance.org/priorities/ingredients/ifra-transparency-list
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/Hydrocinnamic acid, 3,5-di-t-butyl-4-hydroxy-, octadecyl esterhttps://www.epa.govt.nz/industry-areas/hazardous-substances/guidance-for-importers-and-manufacturers/hazardous-substances-databases/
- Consumer Product Information Database (CPID)LICENSECopyright (c) 2024 DeLima Associates. All rights reserved. Unless otherwise indicated, all materials from CPID are copyrighted by DeLima Associates. No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://www.whatsinproducts.com/contents/view/1/6Hydrocinnamic acid, 3,5-di-t-butyl-4-hydroxy-, octadecyl esterhttps://www.whatsinproducts.com/chemicals/view/1/3941/002082-79-3Consumer Products Category Classificationhttps://www.whatsinproducts.com/
- EPA Chemical and Products Database (CPDat)Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionatehttps://comptox.epa.gov/dashboard/DTXSID8027456#exposureEPA CPDat Classificationhttps://www.epa.gov/chemical-research/chemical-and-products-database-cpdat
- Haz-Map, Information on Hazardous Chemicals and Occupational DiseasesLICENSECopyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://haz-map.com/AboutHydrocinnamic acid, 3,5-di-t-butyl-4-hydroxy-, octadecyl esterhttps://haz-map.com/Agents/5560
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- NITE-CMCn-Octadecyl 3-(4'-hydroxy-3',5'-di-t-butylphenyl) propionate - FY2016 (New/original classication)https://www.chem-info.nite.go.jp/chem/english/ghs/16-mhlw-0030e.html
- Japan Chemical Substance Dictionary (Nikkaji)
- MassBank EuropeOCTADECYL 3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATEhttps://massbank.eu/MassBank/Result.jsp?inchikey=SSDSCDGVMJFTEQ-UHFFFAOYSA-N
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/licenseOCTADECYL 3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATEhttps://mona.fiehnlab.ucdavis.edu/spectra/browse?query=exists(compound.metaData.name:%27InChIKey%27%20and%20compound.metaData.value:%27SSDSCDGVMJFTEQ-UHFFFAOYSA-N%27)
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawBenzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, octadecyl esterhttp://www.nist.gov/srd/nist1a.cfm
- SpectraBaseBENZENEPROPANOIC ACID, 3,5-BIS(1,1-DIMETHYLETHYL)-4-HYDROXY-, OCTADECYL ESTERhttps://spectrabase.com/spectrum/3ubpkaHZDW1OCTADECYL 3-(3,5-DI-tert-BUTYL-4-HYDROXYPHENYL) PROPIONATEhttps://spectrabase.com/spectrum/9Z6aDAv1yO7OCTADECYL-3-(3',5'-DI-tert-BUTYL-4'-HYDROXYPHENYL)PROPIONATEhttps://spectrabase.com/spectrum/B5lKCim9Xf6OCTADECYL 3-(3,5-DI-tert-BUTYL-4-HYDROXYPHENYL) PROPIONATEhttps://spectrabase.com/spectrum/4zAY97cjqblOCTADECYL 3-(3',5'-DI-tert-BUTYL-4'-HYDROXYPHENYL)PROPIONATEhttps://spectrabase.com/spectrum/87IM4s5ASSNOctadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionatehttps://spectrabase.com/spectrum/Js76dHI8BsL3,5-Di(T-butyl)-4-hydroxy-benzenepropanoic acid, octadecyl esterhttps://spectrabase.com/spectrum/A7KFuX8VmQQOctadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionatehttps://spectrabase.com/spectrum/3p9jSrztgYZOctadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionatehttps://spectrabase.com/spectrum/8RiENahelY7
- NMRShiftDB
- Springer Nature
- Wikidataoctadecyl di-tert-butyl-4-hydroxyhydrocinnamatehttps://www.wikidata.org/wiki/Q27291662
- WikipediaNeptunium(IV) phosphidehttps://en.wikipedia.org/wiki/Neptunium(IV)_phosphideOctadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionatehttps://en.wikipedia.org/wiki/Octadecyl_3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlIrganox 1076https://www.ncbi.nlm.nih.gov/mesh/67024538
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403395949https://pubchem.ncbi.nlm.nih.gov/substance/403395949