Aminopyrine
- AMINOPYRINE
- aminophenazone
- Amidopyrine
- 58-15-1
- 4-Dimethylaminoantipyrine
- Create:2005-03-26
- Modify:2025-01-18
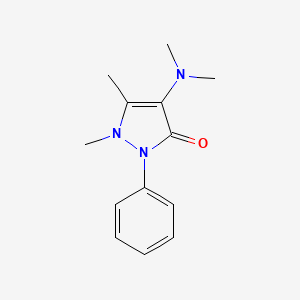
- Amidazophen
- Amidophen
- Amidophenazon
- Amidopyrine
- Aminofenazone
- Aminophenazone
- Aminopyrine
- Dimethyl N aminoantipyrine
- Dimethyl-N-aminoantipyrine
- Dimethylaminoantipyrine
- Dimethylaminophenazone
- Dipyrine
- Eufibron
- AMINOPYRINE
- aminophenazone
- Amidopyrine
- 58-15-1
- 4-Dimethylaminoantipyrine
- Dipyrine
- Aminopyrin
- Amidopyrazoline
- Amidazophene
- Pyramidon
- Aminofenazone
- Amidazophen
- Amidophen
- Brufaneuxol
- Eufibron
- Itamidone
- Pyramidone
- Dereuma
- Febron
- Dimethylaminoazophene
- Amidophenazone
- Aminophenazon
- Dimethylaminoantipyrine
- Amidofebrin
- Amidofen
- Amidopyrin
- Anafebrina
- Netsusarin
- Dimethylaminophenazone
- Amidazofen
- Piromidina
- Dimapyrin
- Dipirin
- Dipyrin
- Febrinina
- Hyparon
- Novamidon
- Piramidon
- Pirazon
- Piridol
- Polinalin
- Pyradone
- 4-(Dimethylamino)antipyrine
- Mamallet-A
- 4-Dimethylaminophenazone
- Amidazophenum
- Aminofenazona
- Aminopyrinum
- 4-Dimethylamino-2,3-dimethyl-1-phenyl-3-pyrazolin-5-one
- Dimethylamino-analgesine
- Dimethylaminophenyldimethylpyrazolone
- Dimethylaminophenazon
- Aminopyrine [JAN]
- Aminophenazonum
- (Dimethylamino)phenazone
- 4-(Dimethylamino)-1,5-dimethyl-2-phenyl-1,2-dihydro-3H-pyrazol-3-one
- Antipyrine, 4-(dimethylamino)-
- 4-Dimethylamino-1-phenyl-2,3-dimethylpyrazolone
- Aminofenazona [INN-Spanish]
- Aminophenazonum [INN-Latin]
- 3H-Pyrazol-3-one, 4-(dimethylamino)-1,2-dihydro-1,5-dimethyl-2-phenyl-
- Aminophenazone [INN]
- 1,5-Dimethyl-4-dimethylamino-2-phenyl-3-pyrazolone
- 1-Phenyl-2,3-dimethyl-4-dimethylaminopyrazol-5-one
- 2,3-Dimethyl-4-dimethylamino-1-phenyl-5-pyrazolone
- 4-Dimethylamino-2,3-dimethyl-1-phenyl-5-pyrazolone
- CCRIS 2907
- Aminopyrine [JAN:NF]
- HSDB 2135
- Mamallet a
- Dimethylaminophenyldimethylpyrazolin
- NSC 4993
- EINECS 200-365-8
- Aminopyrine (JAN)
- UNII-01704YP3MO
- Aminophenazone (INN)
- DTXSID7020504
- 3-Keto-1,5-dimethyl-4-dimethylamino-2-phenyl-2,3-dihydropyrazole
- Dimethylaminophenyldimethylpyrazone
- 1-Phenyl-2,3-dimethyl-4-(dimethylamino)-5-pyrazolone
- NSC-4993
- AMINOPYRINE [MI]
- MFCD00003142
- AMINOPYRINE [HSDB]
- 4-(Dimethylamino)phenazone
- 3H-Pyrazol-3-one,4-(dimethylamino)-1,2-dihydro-1,5-dimethyl-2-phenyl-
- 4-(Dimethylamino)-1,2-dihydro-1,5-dimethyl-2-phenyl-3H-pyrazol-3-one
- 4-(dimethylamino)-1,5-dimethyl-2-phenylpyrazol-3-one
- DTXCID70504
- AMINOPHENAZONE [MART.]
- MLS002154195
- AMINOPHENAZONE [WHO-DD]
- 3-Pyrazolin-5-one, 4-(dimethylamino)-2,3-dimethyl-1-phenyl-
- CHEBI:160246
- 01704YP3MO
- CAS-58-15-1
- NCGC00016257-04
- Amidophenazon
- Amidopyrinum
- SMR001216566
- 4-(dimethylamino)-1,5-dimethyl-2-phenyl-2,3-dihydro-1H-pyrazol-3-one
- 4-Dimethylamino Antipyrine
- Aminofenazona (INN-Spanish)
- Aminophenazonum (INN-Latin)
- 1-Phenyl-2,3-dimethyl-4-dimethylaminopyrazolone-5
- 1,5-Dimethyl-2-phenyl-4-dimethylamino-3-pyrazolone
- AMINOPHENAZONE (MART.)
- Aminophenazon [German]
- METAMIZOLE SODIUM MONOHYDRATE IMPURITY D [EP IMPURITY]
- Aminofenazone [Italian]
- Dimethyl-N-aminoantipyrine
- 4-DIMETHYLAMINO-1,5-DIMETHYL-2-PHENYL-1,2-DIHYDRO-3H-PYRAZOL-3-ONE
- Dimethylaminophenazon [German]
- dimethylaminoanalgesine
- SR-05000001741
- Dimethyl N aminoantipyrine
- Piramidone
- METAMIZOLE SODIUM MONOHYDRATE IMPURITY D (EP IMPURITY)
- Prestwick_14
- Prestwick0_000088
- Prestwick1_000088
- Prestwick2_000088
- Prestwick3_000088
- cid_6009
- TimTec1_000737
- 3-keto-1,3-dihydropyrazole
- Oprea1_080671
- SCHEMBL26293
- BSPBio_000016
- 4-N,N-Dimethylaminoantipyrine
- 4-Dimethylamino-2,3-dimethyl-1-phenylpyrazol-5-one
- SPBio_001955
- BPBio1_000018
- CHEMBL288470
- P-DIMETHYLAMINOANTIPYRINE
- (DIMETHYLAMINO)ANALGESINE
- (DIMETHYLAMINO)ANTIPYRINE
- 4-(dimethylamino)-1,5-dimethyl-2-phenyl-pyrazol-3-one
- BDBM74258
- N02BB03
- NSC4993
- 4-(dimethylamino)-2,3-dimethyl-1-phenyl-3-pyrazolin-5-one
- HMS1536B11
- HMS1568A18
- HMS2092E10
- HMS2095A18
- HMS3039C06
- HMS3652G13
- HMS3712A18
- Pharmakon1600-01500621
- HY-B0533
- Tox21_110333
- Tox21_201544
- Tox21_302830
- NSC757388
- s3209
- STL356033
- AKOS001590378
- Tox21_110333_1
- CCG-103785
- DB01424
- NSC-757388
- NCGC00016257-01
- NCGC00016257-02
- NCGC00016257-03
- NCGC00016257-05
- NCGC00016257-06
- NCGC00016257-07
- NCGC00016257-10
- NCGC00090878-01
- NCGC00090878-02
- NCGC00090878-03
- NCGC00175173-01
- NCGC00175173-02
- NCGC00256436-01
- NCGC00259094-01
- 3-Pyrazolin-5-one,3-dimethyl-1-phenyl-
- AC-12025
- SBI-0207073.P001
- WLN: T5NNVJ A1 BR& DN1&1 E1
- DB-053161
- NS00000319
- SW196332-3
- C07539
- D00556
- EN300-18532179
- Q416503
- SR-05000001741-1
- SR-05000001741-3
- SR-05000001741-4
- 4-(dimethylamino)-1,5-dimethyl-2-phenyl-3-pyrazolone
- BRD-K12568846-001-04-7
- BRD-K12568846-001-14-6
- BRD-K12568846-001-15-3
- BRD-K12568846-001-16-1
- 4-(dimethylamino)-1,5-dimethyl-2-phenyl-3-pyrazolin-3-one
- 4-Dimethylaminoantipyrine, reactive nitrogen species scavenger
- 4-(dimethylamino)-1,5-dimethyl-2-phenyl-1H-pyrazol-3(2H)-one
- 4-(Dimethylamino)-1,5-dimethyl-2-phenyl-1,2-dihydro-3H-pyrazol-3-one #
- pyrazole, 2,3-dihydro-4-dimethylamino-1,5-dimethyl-3-oxo-2-phenyl-
- pyrazole, 4-dimethylamino-2,3-dihydro-1,5-dimethyl-3-oxo-2-phenyl-
- Aminophenazone (4-Dimethylamino-1,5-dimethyl-2-phenyl-1,2-dihydro-3H-pyrazol-3-one)
- 4-Dimethylamino-1,5-dimethyl-2-phenyl-1,2-dihydro-3H-pyrazol-3-one (4,4-Dimethylaminophenazone)
231.0 99.99
56.0 92
97.0 71
111.0 37
232.0 28
56.0 99.99
97.0 35.90
42.0 12.90
111.0 11.90
77.0 8.40
58.0651 100
56.0495 94.49
97.076 94.07
70.0651 47.67
72.0807 26.85
58.0651 100
56.0495 83.97
97.0761 80.51
70.0651 57.28
98.0839 46.92
232.1442 999
233.1468 156
187.0852 34
139.0854 20
159.09 15
232.1438 999
177.101 723
159.0902 582
187.0851 300
149.1059 284
231 999
56 920
97 710
111 370
232 280


H301 (96.4%): Toxic if swallowed [Danger Acute toxicity, oral]
H315 (89.3%): Causes skin irritation [Warning Skin corrosion/irritation]
H319 (89.3%): Causes serious eye irritation [Warning Serious eye damage/eye irritation]
H335 (87.5%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation]
P261, P264, P264+P265, P270, P271, P280, P301+P316, P302+P352, P304+P340, P305+P351+P338, P319, P321, P330, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 56 reports by companies from 8 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Acute Tox. 3 (96.4%)
Skin Irrit. 2 (89.3%)
Eye Irrit. 2 (89.3%)
STOT SE 3 (87.5%)
SYMPTOMS: Symptoms of exposure to this compound include allergic reactions, strong spasmolytic effect on smooth muscle of peripheral blood vessels, irritability, palsy, copious sweating, dilated pupils, sharp drop then rise in body temperature, dysuria, dyspnea, anxiety, tenesmus, urinary frequency, intermittent fever, fatty infiltration of the liver, heart muscle degeneration and death due to circulatory failure following cardiovascular collapse. Agranulocytosis often occurs. Ingestion may cause central nervous system stimulation, vomiting, convulsions, cyanosis, tinnitus, leukopenia, kidney damage and coma. Ingestion may also lead to nausea, mental disturbances, methemoglobinemia, chocolate-colored blood, dizziness, epigastric pain, difficulty in hearing, thready pulse and liver damage. Other symptoms reported via ingestion include hemolytic anemia, porphyria and severe gastrointestinal bleeding. Bone marrow depression also occurs. Rare eye effects include acute transient myopia. Chronic symptoms include anorexia, edema, oliguria, urticaria, hypersensitivity, aplastic anemia, sore throat, fever, pharyngeal membrane, jaundice enlargement of the liver and spleen, exfoliative dermatitis, gastric or duodenal erosion with perforation or bleeding, adrenal necrosis, thrombocytopenic purpura and acute leukemia.
ACUTE/CHRONIC HAZARDS: When heated to decomposition this compound emits toxic fumes of nitrogen oxides. (NTP, 1992)
EYES: First check the victim for contact lenses and remove if present. Flush victim's eyes with water or normal saline solution for 20 to 30 minutes while simultaneously calling a hospital or poison control center. Do not put any ointments, oils, or medication in the victim's eyes without specific instructions from a physician. IMMEDIATELY transport the victim after flushing eyes to a hospital even if no symptoms (such as redness or irritation) develop.
SKIN: IMMEDIATELY flood affected skin with water while removing and isolating all contaminated clothing. Gently wash all affected skin areas thoroughly with soap and water. If symptoms such as redness or irritation develop, IMMEDIATELY call a physician and be prepared to transport the victim to a hospital for treatment.
INHALATION: IMMEDIATELY leave the contaminated area; take deep breaths of fresh air. If symptoms (such as wheezing, coughing, shortness of breath, or burning in the mouth, throat, or chest) develop, call a physician and be prepared to transport the victim to a hospital. Provide proper respiratory protection to rescuers entering an unknown atmosphere. Whenever possible, Self-Contained Breathing Apparatus (SCBA) should be used; if not available, use a level of protection greater than or equal to that advised under Protective Clothing.
INGESTION: DO NOT INDUCE VOMITING. If the victim is conscious and not convulsing, give 1 or 2 glasses of water to dilute the chemical and IMMEDIATELY call a hospital or poison control center. Be prepared to transport the victim to a hospital if advised by a physician. If the victim is convulsing or unconscious, do not give anything by mouth, ensure that the victim's airway is open and lay the victim on his/her side with the head lower than the body. DO NOT INDUCE VOMITING. IMMEDIATELY transport the victim to a hospital. (NTP, 1992)
Excerpt from ERG Guide 154 [Substances - Toxic and/or Corrosive (Non-Combustible)]:
IMMEDIATE PRECAUTIONARY MEASURE: Isolate spill or leak area in all directions for at least 50 meters (150 feet) for liquids and at least 25 meters (75 feet) for solids.
SPILL: Increase the immediate precautionary measure distance, in the downwind direction, as necessary.
FIRE: If tank, rail tank car or highway tank is involved in a fire, ISOLATE for 800 meters (1/2 mile) in all directions; also, consider initial evacuation for 800 meters (1/2 mile) in all directions. (ERG, 2024)
SMALL SPILLS AND LEAKAGE: If you spill this chemical, you should dampen the solid spill material with water, then transfer the dampened material to a suitable container. Use absorbent paper dampened with water to pick up any remaining material. Seal your contaminated clothing and the absorbent paper in a vapor-tight plastic bag for eventual disposal. Wash all contaminated surfaces with a soap and water solution. Do not reenter the contaminated area until the Safety Officer (or other responsible person) has verified that the area has been properly cleaned.
STORAGE PRECAUTIONS: You should protect this material from exposure to light, and store it in a refrigerator. (NTP, 1992)
Amides and Imides
Amines, Phosphines, and Pyridines
Hydrocarbons, Aliphatic Unsaturated
IMAP assessments - 3H-Pyrazol-3-one, 4-(dimethylamino)-1,2-dihydro-1,5-dimethyl-2-phenyl-: Environment tier I assessment
IMAP assessments - 3H-Pyrazol-3-one, 4-(dimethylamino)-1,2-dihydro-1,5-dimethyl-2-phenyl-: Human health tier I assessment
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=RMMXTBMQSGEXHJ-UHFFFAOYSA-N
- Australian Industrial Chemicals Introduction Scheme (AICIS)3H-Pyrazol-3-one, 4-(dimethylamino)-1,2-dihydro-1,5-dimethyl-2-phenyl-https://services.industrialchemicals.gov.au/search-assessments/3H-Pyrazol-3-one, 4-(dimethylamino)-1,2-dihydro-1,5-dimethyl-2-phenyl-https://services.industrialchemicals.gov.au/search-inventory/
- CAMEO ChemicalsLICENSECAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data.https://cameochemicals.noaa.gov/help/reference/terms_and_conditions.htm?d_f=false4-DIMETHYLAMINOANTIPYRINEhttps://cameochemicals.noaa.gov/chemical/20225CAMEO Chemical Reactivity Classificationhttps://cameochemicals.noaa.gov/browse/react
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusAminopyrine [JAN:NF]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000058151ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_useAminophenazonehttps://www.drugbank.ca/drugs/DB01424
- DTP/NCILICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuse
- EPA Chemicals under the TSCA3H-Pyrazol-3-one, 4-(dimethylamino)-1,2-dihydro-1,5-dimethyl-2-phenyl-https://www.epa.gov/chemicals-under-tscaEPA TSCA Classificationhttps://www.epa.gov/tsca-inventory
- EPA DSSTox4-Dimethylaminoantipyrinehttps://comptox.epa.gov/dashboard/DTXSID7020504CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeAminophenazone (EC: 200-365-8)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/125969
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Hazardous Substances Data Bank (HSDB)
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingAminophenazonehttp://www.hmdb.ca/metabolites/HMDB0015493HMDB0015493_cms_27466https://hmdb.ca/metabolites/HMDB0015493#spectra
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/3H-Pyrazol-3-one, 4-(dimethylamino)-1,2-dihydro-1,5-dimethyl-2-phenyl-https://www.epa.govt.nz/industry-areas/hazardous-substances/guidance-for-importers-and-manufacturers/hazardous-substances-databases/
- ChEBI
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licence
- CCSbaseCCSbase Classificationhttps://ccsbase.net/
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jsp
- IUPAC Digitized pKa Datasetpyrazole, 4-dimethylamino-2,3-dihydro-1,5-dimethyl-3-oxo-2-phenyl-https://github.com/IUPAC/Dissociation-Constants
- MassBank Europe
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawAminopyrinehttp://www.nist.gov/srd/nist1a.cfm
- SpectraBaseAminopyrinehttps://spectrabase.com/spectrum/oFpAV5TsJ5Aminopyrinehttps://spectrabase.com/spectrum/BNQhhBNvQvC4-Dimethylaminoantipyrinehttps://spectrabase.com/spectrum/9w37XuJ9FvyAminophenazonehttps://spectrabase.com/spectrum/AzbEA7lL8NF4-(DIMETHYLAMINO)ANTIPYRINEhttps://spectrabase.com/spectrum/329bCduDkqG4-(Dimethylamino)antipyrinehttps://spectrabase.com/spectrum/1KecsBAcjmw4-(dimethylamino)antipyrinehttps://spectrabase.com/spectrum/Hc62NZuzpIH4-(dimethylamino)antipyrinehttps://spectrabase.com/spectrum/KgHh4CtFM74-(DIMETHYLAMINO)ANTIPYRINEhttps://spectrabase.com/spectrum/FqUS1EqNutJ4-Dimethylaminoantipyrinehttps://spectrabase.com/spectrum/4iQfm0NecBb4-(dimethylamino)antipyrinehttps://spectrabase.com/spectrum/CSrXUBPy5Xy4-(dimethylamino)antipyrinehttps://spectrabase.com/spectrum/KggcSaWmRTKAminopyrinehttps://spectrabase.com/spectrum/2Th54U7nxooAminophenazonehttps://spectrabase.com/spectrum/ImMh05na8S2
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlAnatomical Therapeutic Chemical (ATC) classificationhttp://www.genome.jp/kegg-bin/get_htext?br08303.keg
- Metabolomics Workbench
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/4-DimethylaminoantipyrineNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- WHO Anatomical Therapeutic Chemical (ATC) ClassificationLICENSEUse of all or parts of the material requires reference to the WHO Collaborating Centre for Drug Statistics Methodology. Copying and distribution for commercial purposes is not allowed. Changing or manipulating the material is not allowed.https://www.whocc.no/copyright_disclaimer/Aminophenazonehttps://www.whocc.no/atc_ddd_index/?code=N02BB03
- PharmGKBLICENSEPharmGKB data are subject to the Creative Commons Attribution-ShareALike 4.0 license (https://creativecommons.org/licenses/by-sa/4.0/).https://www.pharmgkb.org/page/policiesaminophenazonehttps://www.pharmgkb.org/chemical/PA164748135
- PharosLICENSEData accessed from Pharos and TCRD is publicly available from the primary sources listed above. Please respect their individual licenses regarding proper use and redistribution.https://pharos.nih.gov/aboutaminophenazonehttps://pharos.nih.gov/ligands/55CTRHQHJC51
- Springer Nature
- SpringerMaterials3H-Pyrazol-3-one, 4-(dimethylamino)-1,2-dihydro-1,5-dimethyl-2-phenyl-https://materials.springer.com/substanceprofile/docs/smsid_bgzaosfhsvvzoyjt
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidataaminophenazonehttps://www.wikidata.org/wiki/Q416503
- WikipediaEthyl maltolhttps://en.wikipedia.org/wiki/Ethyl_maltolAminophenazonehttps://en.wikipedia.org/wiki/Aminophenazone
- Wiley
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlAminopyrinehttps://www.ncbi.nlm.nih.gov/mesh/68000632
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403389639https://pubchem.ncbi.nlm.nih.gov/substance/403389639
- NCBI








