Quinine hydrochloride
- C20H24N2O2.ClH
- C20H25ClN2O2
- Quinine hydrochloride
- 130-89-2
- Quinine HCl
- Quinine muriate
- Chinimetten
- Create:2005-08-08
- Modify:2025-01-18
 Quinine hydrochloride dihydrate (annotation moved to).
Quinine hydrochloride dihydrate (annotation moved to).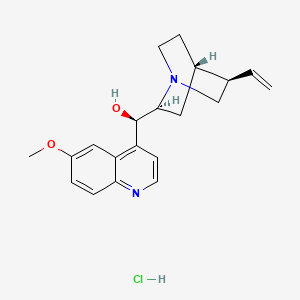
- Biquinate
- Bisulfate, Quinine
- Hydrochloride, Quinine
- Legatrim
- Myoquin
- Quinamm
- Quinbisan
- Quinbisul
- Quindan
- Quinimax
- Quinine
- Quinine Bisulfate
- Quinine Hydrochloride
- Quinine Lafran
- Quinine Sulfate
- Quinine Sulphate
- Quinine-Odan
- Quinoctal
- Quinson
- Quinsul
- Strema
- Sulfate, Quinine
- Sulphate, Quinine
- Surquina
- Quinine hydrochloride
- 130-89-2
- Quinine HCl
- Quinine muriate
- Chinimetten
- Quinine monohydrochloride
- FEMA No. 2976
- CCRIS 2002
- 7549-43-1
- Cinchonan-9-ol, 6'-methoxy-, hydrochloride (1:1), (8alpha,9R)-
- EINECS 205-001-1
- 7CS0WNO31M
- Anhydrous quinine hydrochloride
- Quinine hydrochloride anhydrous
- AI3-62121
- (R)-[(2S,4S,5R)-5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl](6-methoxyquinolin-4-yl)methanol hydrochloride
- (R)-[(2S,4S,5R)-5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl]-(6-methoxyquinolin-4-yl)methanol;hydrochloride
- Chininum muriaticum
- Quinine chloride
- MFCD00078498
- (R)-(6-Methoxyquinolin-4-yl)((1S,2S,4S,5R)-5-vinylquinuclidin-2-yl)methanol hydrochloride
- QUININE, MONOHYDROCHLORIDE
- Quinine hydrochloride hydrate
- Chinine hydrochloride
- Quinine, hydrochloride
- FEMA NO. 2976, DIHYDRATE-
- UNII-7CS0WNO31M
- Cinchonan-9-ol, 6'-methoxy-, monohydrochloride, (8a,9R)- (9CI); Quinine, monohydrochloride (8CI); Chinimetten; Chinine hydrochloride; Quinine hydrochloride; Quinine muriate
- EINECS 231-437-7
- Quinini hydrochloridum
- (8alpha,9R)-6'-Methoxycinchonan-9-ol monohydrochloride
- 6'-Methoxycinchonan-9-ol monohydrochloride, (8alpha,9R)-
- Cinchonan-9-ol, 6'-methoxy-, monohydrochloride, (8alpha,9R)-
- Quinine hydrochloride (1:1)
- SCHEMBL184367
- CHEMBL588046
- DTXSID7044213
- TCMDC-123484
- TCMDC-125479
- Cinchonan-9-ol, 6'-methoxy-, hydrochloride, (8alpha,9R)-
- Cinchonan-9-ol, 6'-methoxy-, hydrochloride, (8.alpha.,9R)-
- Cinchonan-9-ol, 6'-methoxy-, monohydrochloride, (8-alpha,9R)-
- QUININE HYDROCHLORIDE [FHFI]
- AKOS016002029
- Cinchonan-9-ol, 6'-methoxy-, monohydrochloride, dihydrate, (8alpha,9R)-
- QUININE HYDROCHLORIDE [WHO-DD]
- 6'-methoxycinchonan-9-ol hydrochloride
- AS-70722
- QUININE MONOHYDROCHLORIDE ANHYDROUS
- QUININE HYDROCHLORIDE ANHYDROUS [MI]
- NS00019774
- A51181
- EN300-7357005
- Q27268091
- (8.ALPHA.,9R)-6'-METHOXYCINCHONAN-9-OL HYDROCHLORIDE
- Cinchonan-9-ol, 6'-methoxy-, hydrochloride (1:?), (8alpha,9R)-
- (R)-(6-methoxyquinolin-4-yl)((2S,4S,5R)-5-vinylquinuclidin-2-yl)methanol hydrochloride
 Quinine hydrochloride dihydrate (annotation moved to)
Quinine hydrochloride dihydrate (annotation moved to)
H302 (> 99.9%): Harmful if swallowed [Warning Acute toxicity, oral]
H312 (91.4%): Harmful in contact with skin [Warning Acute toxicity, dermal]
H332 (91.4%): Harmful if inhaled [Warning Acute toxicity, inhalation]
P261, P264, P270, P271, P280, P301+P317, P302+P352, P304+P340, P317, P321, P330, P362+P364, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 1409 reports by companies from 7 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Acute Tox. 4 (> 99.9%)
Acute Tox. 4 (91.4%)
Acute Tox. 4 (91.4%)
Acute Tox. 4 (100%)
Acute Tox. 4 (11.1%)
Skin Sens. 1 (66.7%)
Acute Tox. 4 (55.6%)
Resp. Sens. 1 (44.4%)
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=LBSFSRMTJJPTCW-DSXUQNDKSA-N
- Australian Industrial Chemicals Introduction Scheme (AICIS)Cinchonan-9-ol, 6'-methoxy-, monohydrochloride, (8.alpha.,9R)-https://services.industrialchemicals.gov.au/search-assessments/Cinchonan-9-ol, 6'-methoxy-, monohydrochloride, (8.alpha.,9R)-https://services.industrialchemicals.gov.au/search-inventory/
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/Cinchonan-9-ol, 6′-methoxy-, hydrochloride (1:?), (8α,9R)-https://commonchemistry.cas.org/detail?cas_rn=7549-43-1(-)-Quinine dihydrochloridehttps://commonchemistry.cas.org/detail?cas_rn=60-93-5Quinine, monohydrochloridehttps://commonchemistry.cas.org/detail?cas_rn=130-89-2Quinine, hydrochloride (2:1)https://commonchemistry.cas.org/detail?cas_rn=30860-23-2
- ChemIDplusQuinine hydrochloridehttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000130892Quinine hydrochloridehttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0007549431ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA Chemicals under the TSCACinchonan-9-ol, 6'-methoxy-, hydrochloride (1:1), (8.alpha.,9R)-https://www.epa.gov/chemicals-under-tscaEPA TSCA Classificationhttps://www.epa.gov/tsca-inventory
- EPA DSSToxQuinine hydrochloride (1:1)https://comptox.epa.gov/dashboard/DTXSID7044213CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeQuinine hydrochloridehttps://echa.europa.eu/substance-information/-/substanceinfo/100.028.580(8α,9R)-6'-methoxycinchonan-9-ol monohydrochloridehttps://echa.europa.eu/substance-information/-/substanceinfo/100.004.548(8α,9R)-6'-methoxycinchonan-9-ol monohydrochloride (EC: 205-001-1)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/34845Quinine hydrochloride (EC: 231-437-7)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/120188
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingQUININE HYDROCHLORIDE ANHYDROUShttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/7CS0WNO31M
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/Cinchonan-9-ol, 6'-methoxy-, monohydrochloride, (8.alpha.,9R)-https://www.epa.govt.nz/industry-areas/hazardous-substances/guidance-for-importers-and-manufacturers/hazardous-substances-databases/
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- EPA Chemical and Products Database (CPDat)Quinine hydrochloride (1:1)https://comptox.epa.gov/dashboard/DTXSID7044213#exposureEPA CPDat Classificationhttps://www.epa.gov/chemical-research/chemical-and-products-database-cpdat
- EU Food Improvement AgentsQuinine hydrochloridehttps://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32012R0872
- Joint FAO/WHO Expert Committee on Food Additives (JECFA)LICENSEPermission from WHO is not required for the use of WHO materials issued under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Intergovernmental Organization (CC BY-NC-SA 3.0 IGO) licence.https://www.who.int/about/policies/publishing/copyrightQUININE HYDROCHLORIDEhttps://apps.who.int/food-additives-contaminants-jecfa-database/Home/Chemical/933
- Hazardous Chemical Information System (HCIS), Safe Work Australia
- FDA Substances Added to FoodLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Flavor and Extract Manufacturers Association (FEMA)QUININE HYDROCHLORIDEhttps://www.femaflavor.org/flavor-library/quinine-hydrochloride
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingQuinine hydrochloridehttp://www.hmdb.ca/metabolites/HMDB0302140
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.html
- National Drug Code (NDC) DirectoryLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingQUININE HYDROCHLORIDEhttps://www.fda.gov/drugs/drug-approvals-and-databases/national-drug-code-directory
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- NIPH Clinical Trials Search of Japan
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licenceQUININE HYDROCHLORIDEhttps://platform.opentargets.org/drug/CHEMBL588046
- SpectraBaseQUININE HYDROCHLORIDEhttps://spectrabase.com/spectrum/HqgxXGRABTC
- Springer Nature
- Wikidataquinine hydrochloridehttps://www.wikidata.org/wiki/Q27268091
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlMuscle Relaxants, Centralhttps://www.ncbi.nlm.nih.gov/mesh/68009125Analgesics, Non-Narcotichttps://www.ncbi.nlm.nih.gov/mesh/68018712Antimalarialshttps://www.ncbi.nlm.nih.gov/mesh/68000962
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- Consumer Product Information Database (CPID)LICENSECopyright (c) 2024 DeLima Associates. All rights reserved. Unless otherwise indicated, all materials from CPID are copyrighted by DeLima Associates. No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://www.whatsinproducts.com/contents/view/1/6Consumer Products Category Classificationhttps://www.whatsinproducts.com/
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403430547https://pubchem.ncbi.nlm.nih.gov/substance/403430547
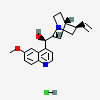

 CID 3034034 (Quinine)
CID 3034034 (Quinine) CID 313 (Hydrochloric Acid)
CID 313 (Hydrochloric Acid)