Aspartate potassium
PubChem CID
71586907
Molecular Formula
Synonyms
- Aspartate potassium
- Potassium aspartate
- L-Aspartate potassium
- UNII-OC4598NZEQ
- OC4598NZEQ
Molecular Weight
360.40 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Parent Compound
Dates
- Create:2013-07-08
- Modify:2025-01-18
Description
One of the non-essential amino acids commonly occurring in the L-form. It is found in animals and plants, especially in sugar cane and sugar beets. It may be a neurotransmitter.
Chemical Structure Depiction
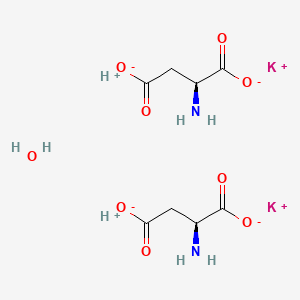
3D Conformer of Parent
SVG Image

IUPAC Condensed
H-Asp-OH.H-Asp-OH.2K+.H2O
IUPAC
L-aspartic acid compound with L-aspartic acid; hydrate; potassium salt
dipotassium;(2S)-2-aminobutanedioate;hydron;hydrate
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/2C4H7NO4.2K.H2O/c2*5-2(4(8)9)1-3(6)7;;;/h2*2H,1,5H2,(H,6,7)(H,8,9);;;1H2/q;;2*+1;/p-2/t2*2-;;;/m00.../s1
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
YKZPPPNXRZHVGX-PXYKVGKMSA-L
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
[H+].[H+].C([C@@H](C(=O)[O-])N)C(=O)[O-].C([C@@H](C(=O)[O-])N)C(=O)[O-].O.[K+].[K+]
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C8H14K2N2O9
Computed by PubChem 2.2 (PubChem release 2021.10.14)
7259-25-8
14434-35-6
- (+-)-Aspartic Acid
- (R,S)-Aspartic Acid
- Ammonium Aspartate
- Aspartate
- Aspartate Magnesium Hydrochloride
- Aspartate, Ammonium
- Aspartate, Calcium
- Aspartate, Dipotassium
- Aspartate, Disodium
- Aspartate, Magnesium
- Aspartate, Monopotassium
- Aspartate, Monosodium
- Aspartate, Potassium
- Aspartate, Sodium
- Aspartic Acid
- Aspartic Acid, Ammonium Salt
- Aspartic Acid, Calcium Salt
- Aspartic Acid, Dipotassium Salt
- Aspartic Acid, Disodium Salt
- Aspartic Acid, Hydrobromide
- Aspartic Acid, Hydrochloride
- Aspartic Acid, Magnesium (1:1) Salt, Hydrochloride, Trihydrate
- Aspartic Acid, Magnesium (2:1) Salt
- Aspartic Acid, Magnesium-Potassium (2:1:2) Salt
- Aspartic Acid, Monopotassium Salt
- Aspartic Acid, Monosodium Salt
- Aspartic Acid, Potassium Salt
- Aspartic Acid, Sodium Salt
- Calcium Aspartate
- Dipotassium Aspartate
- Disodium Aspartate
- L Aspartate
- L Aspartic Acid
- L-Aspartate
- L-Aspartic Acid
- Magnesiocard
- Magnesium Aspartate
- Mg-5-Longoral
- Monopotassium Aspartate
- Monosodium Aspartate
- Potassium Aspartate
- Sodium Aspartate
- Aspartate potassium
- Potassium aspartate
- L-Aspartate potassium
- UNII-OC4598NZEQ
- OC4598NZEQ
- L-Aspartate potassium [JAN]
- 7259-25-8
- Potassium aspartate hemihydrate
- Aspartic acid, monopotassium salt, hemihydrate, L-
- POTASSIUM ASPARTATE [MART.]
- POTASSIUM ASPARTATE [WHO-DD]
- MONOPOTASSIUM ASPARTATE HEMIHYDRATE
- POTASSIUM HYDROGEN ASPARTATE HEMIHYDRATE
- L-ASPARTIC ACID, MONOPOTASSIUM SALT, HYDRATE (2:1)
- POTASSIUM HYDROGEN ASPARTATE HEMIHYDRATE [EP MONOGRAPH]
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
360.40 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
5
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
11
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
2
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
359.9973430 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
359.9973430 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
214 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
21
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
122
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
2
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
7
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Cosmetic ingredients (Potassium Aspartate) -> CIR (Cosmetic Ingredient Review)
Skin conditioning
S13 | EUCOSMETICS | Combined Inventory of Ingredients Employed in Cosmetic Products (2000) and Revised Inventory (2006) | DOI:10.5281/zenodo.2624118
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Same Parent, Stereo Count
Same Parent, Isotope Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Same Count
Cosmetic Ingredient Review Link
CIR ingredient: Potassium Aspartate
Cosmetics -> Skin conditioning
S13 | EUCOSMETICS | Combined Inventory of Ingredients Employed in Cosmetic Products (2000) and Revised Inventory (2006) | DOI:10.5281/zenodo.2624118
Household & Commercial/Institutional Products
Information on 1 consumer products that contain Potassium aspartate in the following categories is provided:
• Personal Care
The Australian Inventory of Industrial Chemicals
Chemical: L-Aspartic acid, monopotassium salt, hemihydrate
New Zealand EPA Inventory of Chemical Status
Potassium Aspartate: Does not have an individual approval but may be used as a component in a product covered by a group standard. It is not approved for use as a chemical in its own right.
Chemical Assessment
IMAP assessments - L-Aspartic acid, monopotassium salt, hemihydrate: Environment tier I assessment
Evaluation - Chemicals that are unlikely to require further regulation to manage risks to human health
- Australian Industrial Chemicals Introduction Scheme (AICIS)L-Aspartic acid, monopotassium salt, hemihydratehttps://services.industrialchemicals.gov.au/search-assessments/L-Aspartic acid, monopotassium salt, hemihydratehttps://services.industrialchemicals.gov.au/search-inventory/
- ChemIDplusPotassium aspartatehttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0007259258ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_usePotassium aspartatehttps://www.drugbank.ca/drugs/DB15819
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingPOTASSIUM ASPARTATEhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/OC4598NZEQ
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/
- Consumer Product Information Database (CPID)LICENSECopyright (c) 2024 DeLima Associates. All rights reserved. Unless otherwise indicated, all materials from CPID are copyrighted by DeLima Associates. No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://www.whatsinproducts.com/contents/view/1/6Potassium aspartatehttps://www.whatsinproducts.com/chemicals/view/1/3761/014007-45-5Consumer Products Category Classificationhttps://www.whatsinproducts.com/
- Cosmetic Ingredient Review (CIR)
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.html
- National Drug Code (NDC) DirectoryLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.htmlpotassium aspartatehttps://rxnav.nlm.nih.gov/id/rxnorm/8589
- WikidataPotassium aspartate hemihydratehttps://www.wikidata.org/wiki/Q81977329
- WikipediaPotassium aspartatehttps://en.wikipedia.org/wiki/Potassium_aspartate
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlAspartic Acidhttps://www.ncbi.nlm.nih.gov/mesh/68001224
- PubChem
CONTENTS

 CID 5960 (Aspartic Acid)
CID 5960 (Aspartic Acid) CID 783 (Hydrogen)
CID 783 (Hydrogen) CID 5462222 (Potassium)
CID 5462222 (Potassium) CID 962 (Water)
CID 962 (Water)