Phenytoin calcium
- Phenytoin calcium
- Calcium Phenytoin
- UNII-9C9H20H712
- EINECS 241-241-3
- 9C9H20H712
- Create:2019-01-15
- Modify:2025-01-10
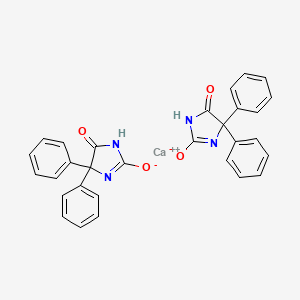
- 5,5-Diphenylhydantoin
- 5,5-diphenylimidazolidine-2,4-dione
- Antisacer
- Difenin
- Dihydan
- Dilantin
- Diphenylhydantoin
- Diphenylhydantoinate, Sodium
- Epamin
- Epanutin
- Fenitoin
- Hydantol
- Phenhydan
- Phenytoin
- Phenytoin Sodium
- Sodium Diphenylhydantoinate

H302 (100%): Harmful if swallowed [Warning Acute toxicity, oral]
H312 (100%): Harmful in contact with skin [Warning Acute toxicity, dermal]
H332 (100%): Harmful if inhaled [Warning Acute toxicity, inhalation]
H351 (100%): Suspected of causing cancer [Warning Carcinogenicity]
P203, P261, P264, P270, P271, P280, P301+P317, P302+P352, P304+P340, P317, P318, P321, P330, P362+P364, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-notice5,5-diphenylimidazolidine-2,4-dione, calcium salt (2:1)https://echa.europa.eu/substance-information/-/substanceinfo/100.037.4775,5-diphenylimidazolidine-2,4-dione, calcium salt (2:1) (EC: 241-241-3)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/94880
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingPHENYTOIN CALCIUMhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/9C9H20H712
- Wikidataphenytoin calciumhttps://www.wikidata.org/wiki/Q27272348
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlAnticonvulsantshttps://www.ncbi.nlm.nih.gov/mesh/68000927Cytochrome P-450 CYP1A2 Inducershttps://www.ncbi.nlm.nih.gov/mesh/68065694Voltage-Gated Sodium Channel Blockershttps://www.ncbi.nlm.nih.gov/mesh/68061567
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- NCBI

 CID 1775 (Phenytoin)
CID 1775 (Phenytoin) CID 5460341 (Calcium)
CID 5460341 (Calcium)