Levocarnitine
- L-carnitine
- Levocarnitine
- 541-15-1
- (R)-Carnitine
- Carnitor
- Create:2005-06-01
- Modify:2025-01-18
 Acetyl-L-carnitine hydrochloride (is active moiety of);
Acetyl-L-carnitine hydrochloride (is active moiety of);  Levocarnitine Propionate Hydrochloride (is active moiety of) ... View More ...
Levocarnitine Propionate Hydrochloride (is active moiety of) ... View More ...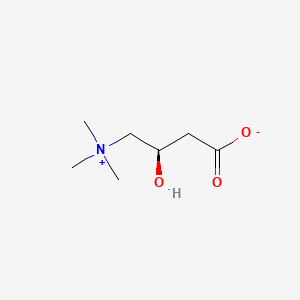
- Bicarnesine
- Carnitine
- L Carnitine
- L-Carnitine
- Levocarnitine
- Vitamin BT
- L-carnitine
- Levocarnitine
- 541-15-1
- (R)-Carnitine
- Carnitor
- vitamin BT
- (-)-Carnitine
- Karnitin
- (-)-L-Carnitine
- L-(-)-Carnitine
- Levocarnitina
- Carnitene
- Metina
- Carnitine, (-)-
- bicarnesine
- Levocarnitinum
- Carnitolo
- Carnovis
- L-Carnitine inner salt
- Lefcar
- Carniking
- Carnilean
- Carnitor SF
- Levocarnitinum [Latin]
- Levocarnitina [Spanish]
- gamma-Trimethyl-beta-hydroxybutyrobetaine
- (3R)-3-hydroxy-4-(trimethylammonio)butanoate
- vitamin B T
- Carniking 50
- Carnitine, L-
- gamma-Trimethyl-ammonium-beta-hydroxybutirate
- (3R)-3-hydroxy-4-(trimethylazaniumyl)butanoate
- 1-CARNITINE
- 3-Carboxy-2-hydroxy-N,N,N-trimethyl-1-propanaminium hydroxide, inner salt
- L-gamma-trimethyl-beta-hydroxybutyrobetaine
- R-(-)-3-hydroxy-4-trimethylaminobutyrate
- DRG-0211
- 3-carboxy-2-hydroxy-N,N,N-trimethyl-1-propanaminium
- 44985-71-9
- EINECS 208-768-0
- (R)-3-hydroxy-4-(trimethylammonio)butanoate
- UNII-0G389FZZ9M
- (R)-(3-Carboxy-2-hydroxypropyl)trimethylammonium hydroxide
- NSC-759132
- 0G389FZZ9M
- CHEBI:16347
- (L-3-Carboxy-2-hydroxypropyl)trimethylammonium hydroxide, inner salt
- HSDB 7588
- levocarnitine HCl
- 1-Propanaminium, 3-carboxy-2-hydroxy-N,N,N-trimethyl-, inner salt, (R)-
- 1-Propanaminium, 3-carboxy-2-hydroxy-N,N,N-trimethyl-, hydroxide, inner salt, (R)-
- (3-Carboxy-2-hydroxypropyl)trimethyl-ammonium hydroxide, inner salt
- CHEMBL1149
- Ammonium, (3-carboxy-2-hydroxypropyl)trimethyl-, hydroxide, inner salt, L-
- Carnicor
- Carrier
- DTXSID4023208
- (-)-(R)-3-Hydroxy-4-(trimethylammonio)butyrate
- L-carnitine Base
- 1-Propanaminium, 3-carboxy-2-hydroxy-N,N,N-trimethyl-, hydroxide, inner salt
- 1-Propanaminium, 3-carboxy-2-hydroxy-N,N,N-trimethyl-, inner salt, (2R)-
- Levocarnitine [USAN:USP:INN:BAN]
- Oristar lch
- NSC 759132
- Carnitine HCl
- Levocarnitinum (Latin)
- gamma-L-trimethyl-beta-hydroxybutyrobetaine
- (2r)-3-carboxy-2-hydroxy-n,n,n-trimethyl-1-propanaminium inner salt
- LEVOCARNITINE (MART.)
- LEVOCARNITINE [MART.]
- Levocarnitine [USAN:INN]
- LEVOCARNITINE (USP-RS)
- LEVOCARNITINE [USP-RS]
- LEVOCARNITINE (EP MONOGRAPH)
- LEVOCARNITINE [EP MONOGRAPH]
- L Carnitine
- Levocarnitine (USAN:USP:INN:BAN)
- LEVOCARNITINE (USP MONOGRAPH)
- LEVOCARNITINE [USP MONOGRAPH]
- Carnitor (TN)
- SMR000112475
- carnitine (L-form)
- gamma-Trimethyl-hydroxybutyrobetaine
- Levocarnitine;Vitamin B(T)
- 3-hydroxy-4-trimethylammoniobutanoate
- Levocarnil
- Levocarnitin
- Nefrocarnit
- Carnipass
- delta-carnitine
- Levocarnitine (JAN/USP/INN)
- L-Carnitin
- Carnipass 20
- L-Carn
- L-Carnitine,(S)
- 1-Propanaminium,3-carboxy-2-hydroxy-N,N,N-trimethyl-
- CARNITINE HYDROCHLORIDE, DL-
- LEVOCARNITINE SF
- (R)-3-Hydroxy-4-trimethylammoniobutyrate
- L-Carnitine (Standard)
- CARNITINE [MI]
- (R)-3-Hydroxy-4-(trimethylammonio)butyrate
- bmse000211
- L-CARNITINE [FCC]
- Levocarnitine Oral Solution
- C-1985
- L-Carnitin 100 microg/mL in Acetonitrile
- LEVOCARNITINE [INN]
- LEVOCARNITINE [JAN]
- L-carnitine (Levocarnitine)
- L-CARNITINE [VANDF]
- LEVOCARNITINE [HSDB]
- LEVOCARNITINE [USAN]
- SCHEMBL21970
- LEVOCARNITINE [VANDF]
- MLS001332549
- MLS001332550
- BIDD:GT0603
- (3R)-(-)-3-Hydroxy-4-(trimethylammonio)butanoate
- Carnitine dl-form hydrochloride
- LEVOCARNITINE [WHO-DD]
- DTXCID903208
- GTPL4780
- HY-B0399R
- A16AA01
- HMS2093J13
- HMS2267H24
- Pharmakon1600-01505437
- LEVOCARNITINE [ORANGE BOOK]
- HY-B0399
- (R)-(3-Carboxy-2-hydroxypropyl)trimethylammonium hydroxide, inner salt
- Ammonium, (3-carboxy-2-hydroxypropyl)trimethyl-, hydroxide,inner salt
- BDBM50037268
- c0049
- NSC741806
- NSC759132
- s2388
- AKOS005267245
- 1ST1534
- BCP9000830
- CCG-213241
- DB00583
- KS-1422
- NSC-741806
- 3-Hydroxy-4-(trimethylammonio) butanoate
- 3-hydroxy-4-trimethylammoniobutanoic acid
- AS-11974
- (R)-3-Hydroxy-4-trimethylammoniobutanoate
- L-Carnitine inner salt, synthetic, >=98%
- NS00076300
- C00318
- D02176
- AB00919083_05
- AB00919083_06
- EN300-6495783
- beta-Hydroxy-gamma-N,N,N-trimethylammoniobutyrate
- Levocarnitine Oral Solution (Suguar Free) 1 g/10 mL
- Q20735709
- R(-)-beta-Hydroxy-gamma-(N,N,N-trimethylammonio)butyrate
- (-)-(R)-3-Hydroxy-4-(trimethylammonio)butyrate, Vitamin BT
- Levocarnitine, European Pharmacopoeia (EP) Reference Standard
- Levocarnitine, United States Pharmacopeia (USP) Reference Standard
- 1-Propanaminium, 3-carboxy-2-hydroxy-N,N,N-trimethyl-, inner salt, (2R)- (9CI)
- Ammonium, (3-carboxy-2-hydroxypropyl)trimethyl-, hydroxide, inner salt, L- (8CI)
- Levocarnitine, Pharmaceutical Secondary Standard; Certified Reference Material
60.0808 999
43.0184 963
103.0377 782
85.0279 766
102.0904 554
58.0651 999
43.0181 892
60.0806 316
44.0497 257
45.0571 244
184.0937 999
162.1118 897
125.0207 123
Acetyl-L-carnitine hydrochloride (is active moiety of)
Levocarnitine Propionate Hydrochloride (is active moiety of)
(R)-3-Carboxy-2-hydroxy-N,N,N-trimethylpropan-1-aminium (2R,3R)-2,3-dihydroxysuccinate (is active moiety of)
- Arginine; choline hydroxide; levocarnitine; ornithine; pantothenic acid (component of)
- Arginine; glucosamine sulfate; levocarnitine; ornithine; vanadium (component of)
- Cyanocobalamin; human chorionic gonadotropin; levocarnitine; ornithine (component of)
- Artemisia abrotanum flowering top; beef heart; digitalis; egg phospholipids; iodine; levocarnitine; plantago major; potassium chloride; potassium phosphate, dibasic; selenicereus grandiflorus stem; spigelia anthelmia; sus scrofa thyroid; valine (component of)
Use (kg; approx.) in Germany (2009): >1000
Consumption (g per capita; approx.) in Germany (2009): 0.0122
Excretion rate: 0.09
Calculated removal (%): 92.1

H315 (90.5%): Causes skin irritation [Warning Skin corrosion/irritation]
H319 (90.5%): Causes serious eye irritation [Warning Serious eye damage/eye irritation]
P264, P264+P265, P280, P302+P352, P305+P351+P338, P321, P332+P317, P337+P317, and P362+P364
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 1493 reports by companies from 15 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Reported as not meeting GHS hazard criteria per 142 of 1493 reports by companies. For more detailed information, please visit ECHA C&L website.
There are 13 notifications provided by 1351 of 1493 reports by companies with hazard statement code(s).
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Skin Irrit. 2 (90.5%)
Eye Irrit. 2 (90.5%)
Status: Active Update: 04-05-2020 https://echa.europa.eu/registration-dossier/-/registered-dossier/30871
Status: Active Update: 24-01-2019 https://echa.europa.eu/registration-dossier/-/registered-dossier/20814
M Chen, V Vijay, Q Shi, Z Liu, H Fang, W Tong. FDA-Approved Drug Labeling for the Study of Drug-Induced Liver Injury, Drug Discovery Today, 16(15-16):697-703, 2011. PMID:21624500 DOI:10.1016/j.drudis.2011.05.007
M Chen, A Suzuki, S Thakkar, K Yu, C Hu, W Tong. DILIrank: the largest reference drug list ranked by the risk for developing drug-induced liver injury in humans. Drug Discov Today 2016, 21(4): 648-653. PMID:26948801 DOI:10.1016/j.drudis.2016.02.015
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=PHIQHXFUZVPYII-ZCFIWIBFSA-N
- Australian Industrial Chemicals Introduction Scheme (AICIS)1-Propanaminium, 3-carboxy-2-hydroxy-N,N,N-trimethyl-, inner salt, (2R)-https://services.industrialchemicals.gov.au/search-assessments/1-Propanaminium, 3-carboxy-2-hydroxy-N,N,N-trimethyl-, inner salt, (2R)-https://services.industrialchemicals.gov.au/search-inventory/
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusLevocarnitine [USAN:USP:INN:BAN]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000541151ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_useLevocarnitinehttps://www.drugbank.ca/drugs/DB00583
- DTP/NCILICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuse
- EPA DSSToxLevocarnitinehttps://comptox.epa.gov/dashboard/DTXSID4023208CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-notice(R)-(3-carboxy-2-hydroxypropyl)trimethylammonium hydroxidehttps://chem.echa.europa.eu/100.007.972(R)-(3-carboxy-2-hydroxypropyl)trimethylammonium hydroxide (EC: 208-768-0)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/116234
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Hazardous Substances Data Bank (HSDB)
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/
- CCSbaseCCSbase Classificationhttps://ccsbase.net/
- ChEBI
- E. coli Metabolome Database (ECMDB)
- FDA Pharm ClassesLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingFDA Pharmacological Classificationhttps://www.fda.gov/ForIndustry/DataStandards/StructuredProductLabeling/ucm162549.htm
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/Levocarnitinehttps://www.wikidata.org/wiki/Q20735709LOTUS Treehttps://lotus.naturalproducts.net/
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licenceLEVOCARNITINEhttps://platform.opentargets.org/drug/CHEMBL1149
- Toxin and Toxin Target Database (T3DB)LICENSET3DB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (T3DB) and the original publication.http://www.t3db.ca/downloadsL-Carnitinehttp://www.t3db.ca/toxins/T3D2832
- Yeast Metabolome Database (YMDB)(R)-Carnitinehttps://www.ymdb.ca/compounds/YMDB00616
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jsp
- Therapeutic Target Database (TTD)Levacecarnine hcihttps://idrblab.net/ttd/data/drug/details/D0G8SQ
- Cosmetic Ingredient Review (CIR)
- EPA Chemical and Products Database (CPDat)EPA CPDat Classificationhttps://www.epa.gov/chemical-research/chemical-and-products-database-cpdat
- Haz-Map, Information on Hazardous Chemicals and Occupational DiseasesLICENSECopyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://haz-map.com/AboutL-Carnitinehttps://haz-map.com/Agents/15089
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/LEVOCARNITINENORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- Crystallography Open Database (COD)LICENSEAll data in the COD and the database itself are dedicated to the public domain and licensed under the CC0 License. Users of the data should acknowledge the original authors of the structural data.https://creativecommons.org/publicdomain/zero/1.0/
- DailyMed
- Drug Induced Liver Injury Rank (DILIrank) DatasetLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Drugs@FDALICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingLEVOCARNITINEhttps://www.accessdata.fda.gov/scripts/cder/daf/
- ECI Group, LCSB, University of Luxembourg(R)-carnitine
- KNApSAcK Species-Metabolite Database
- Natural Product Activity and Species Source (NPASS)
- West Coast Metabolomics Center-UC DavisCarnitine
- EU Clinical Trials Register
- FDA Orange BookLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- National Drug Code (NDC) DirectoryLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- FDA Substances Added to FoodLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlTherapeutic category of drugs in Japanhttp://www.genome.jp/kegg-bin/get_htext?br08301.kegAnatomical Therapeutic Chemical (ATC) classificationhttp://www.genome.jp/kegg-bin/get_htext?br08303.kegRisk category of Japanese OTC drugshttp://www.genome.jp/kegg-bin/get_htext?br08312.keg
- MarkerDBLICENSEThis work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.https://markerdb.ca/L-Carnitinehttps://markerdb.ca/chemicals/43
- MassBank EuropeL-Carnitine hydrochloridehttps://massbank.eu/MassBank/Result.jsp?inchikey=PHIQHXFUZVPYII-ZCFIWIBFSA-N
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- Metabolomics Workbench
- NIPH Clinical Trials Search of Japan
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawL-Carnitinehttp://www.nist.gov/srd/nist1a.cfm
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.htmllevocarnitinehttps://rxnav.nlm.nih.gov/id/rxnorm/42955
- WHO Anatomical Therapeutic Chemical (ATC) ClassificationLICENSEUse of all or parts of the material requires reference to the WHO Collaborating Centre for Drug Statistics Methodology. Copying and distribution for commercial purposes is not allowed. Changing or manipulating the material is not allowed.https://www.whocc.no/copyright_disclaimer/Levocarnitinehttps://www.whocc.no/atc_ddd_index/?code=A16AA01
- PharmGKBLICENSEPharmGKB data are subject to the Creative Commons Attribution-ShareALike 4.0 license (https://creativecommons.org/licenses/by-sa/4.0/).https://www.pharmgkb.org/page/policiesl-carnitinehttps://www.pharmgkb.org/chemical/PA450154
- Rhea - Annotated Reactions DatabaseLICENSERhea has chosen to apply the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/). This means that you are free to copy, distribute, display and make commercial use of the database in all legislations, provided you credit (cite) Rhea.https://www.rhea-db.org/help/license-disclaimer
- SpectraBase(R)-CARNITINEhttps://spectrabase.com/spectrum/FhWjAWl3n68L-Carnitine inner salthttps://spectrabase.com/spectrum/FPqggC0lksyL(-)-Carnitinehttps://spectrabase.com/spectrum/F53tXBHOJNlL(-)-Carnitinehttps://spectrabase.com/spectrum/3yInlxirDNFL(-)-Carnitinehttps://spectrabase.com/spectrum/AsX6exRtvG4L-Carnitine inner salthttps://spectrabase.com/spectrum/KmoW8FqUdhHL-Carnitine inner salthttps://spectrabase.com/spectrum/K8uGYIMeuyX
- Springer Nature
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- WikidataL-carnitinehttps://www.wikidata.org/wiki/Q20735709
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.html
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403459033https://pubchem.ncbi.nlm.nih.gov/substance/403459033
- NCBI
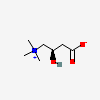

 CID 10918 ((3-Carboxy-2-(R)-hydroxy-propyl)-trimethyl-ammonium)
CID 10918 ((3-Carboxy-2-(R)-hydroxy-propyl)-trimethyl-ammonium)


