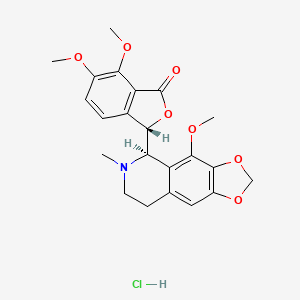
Noscapine Hydrochloride
- C22H23NO7.ClH
- C22H24ClNO7
- Noscapine hydrochloride
- Noscapine HCl
- 912-60-7
- Noscapine hydrchloride
- Narcotine hydrochloride
- Create:2006-10-25
- Modify:2025-01-04
- Capval
- Capval Tropfen
- Embonate, Noscapine Hydrogen
- Hydrochloride, Noscapine
- Hydrogen Embonate, Noscapine
- Librochin prikkelhoest
- Narcotine
- Noscapect
- Noscapine
- Noscapine Hydrochloride
- Noscapine Hydrogen Embonate
- prikkelhoest, Librochin
- Tropfen, Capval
- Tuscalman
- Noscapine hydrochloride
- Noscapine HCl
- 912-60-7
- Noscapine hydrchloride
- Narcotine hydrochloride
- Noscapine (hydrochloride)
- Noscapine (as hydrochloride)
- Teletux
- Tucotine
- Stilco
- Lyobex retard
- Difmetus Kapseln
- Tussicure P
- Germose Noscapine
- Opian hydrochloride
- Noscapine hydrochloride hydrate
- TTN62ITH9I
- Vadebex hydrochloride
- Coscopin hydrochloride
- Nectadon hydrochloride
- NSC-757235
- Opianine hydrochloride
- Terbenol hydrochloride
- Coscotabs hydrochloride
- Narcosine hydrochloride
- Narcompren hydrochloride
- Narcotine, hydrochloride
- DTXSID6073239
- Noscapine hydrochloride anhydrous
- UNII-TTN62ITH9I
- NSC 5366 hydrochloride
- Key-tusscapine hydrochloride
- NOSCAPINE HYDROCHLORIDE [MI]
- Methoxyhydrastine hydrochloride
- CCRIS 8985
- 1-alpha-Narcotine hydrochloride
- NOSCAPINE HYDROCHLORIDE [MART.]
- NOSCAPINE HYDROCHLORIDE [WHO-DD]
- Noscapine hydrochloride [JAN:NF]
- EINECS 213-014-9
- NOSCAPINE HYDROCHLORIDE [EP IMPURITY]
- Noscapine.HCl (Narcotine)
- NOSCAPINE HYDROCHLORIDE ANHYDROUS [WHO-IP]
- (S)-6,7-dimethoxy-3-((R)-4-methoxy-6-methyl-5,6,7,8-tetrahydro-[1,3]dioxolo[4,5-g]isoquinolin-5-yl)isobenzofuran-1(3H)-one hydrochloride
- NOSCAPINI HYDROCHLORIDUM ANHYDROUS [WHO-IP LATIN]
- NOSCAPINE HYDROCHLORIDE (MART.)
- NOSCAPINE HYDROCHLORIDE (EP IMPURITY)
- SR-01000075529
- Opianine hydrochloride; l-Narcotine hydrochloride
- Hydrochloride, Noscapine
- (3S)-6,7-dimethoxy-3-[(5R)-4-methoxy-6-methyl-5,6,7,8-tetrahydro-[1,3]dioxolo[4,5-g]isoquinolin-6-ium-5-yl]-3H-2-benzofuran-1-one;chloride
- 1(3H)Isobenzofuranone, 6,7-dimethoxy-3-(5,6,7,8-tetrahydro-4-methoxy-6-methyl-1,3-dioxolo(4,5-g)isoquinolin-5-yl)-, hydrochloride, (S-(R*,S*))-
- SCHEMBL177962
- CHEMBL2106732
- DTXCID3048730
- Narcotine, hydrochloride (8CI)
- MFLVZFXCSKVCSH-URBRKQAFSA-N
- Tox21_500840
- HY-13716A
- MFCD00149223
- AKOS024282587
- CCG-222144
- LP00840
- NCGC00261525-01
- NOSCAPINI HYDROCHLORIDUM ANHYDROUS
- 1ST10132
- DA-66194
- CS-0013135
- EU-0100840
- N0963
- NS00079565
- N 9007
- SR-01000075529-1
- SR-01000075529-3
- Q27290347
- Noscapine.HCl (Narcotine), 1mg/ml in Methanol (as free base)
- (3S)-6,7-dimethoxy-3-[(5R)-4-methoxy-6-methyl-7,8-dihydro-5H-[1,3]dioxolo[4,5-g]isoquinolin-5-yl]-3H-2-benzofuran-1-one;hydrochloride
- 1(3H)Isobenzofuranone,6,7-dimethoxy-3-(5,6,7,8-tetrahydro-4-methoxy-6-methyl-1,3,-dioxolo(4,5-g)isoquinolin-5-yl)-hydrochloride

P264, P270, P301+P317, P330, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 48 reports by companies from 4 notifications to the ECHA C&L Inventory.
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
IMAP assessments - 1(3H)-Isobenzofuranone, 6,7-dimethoxy-3-(5,6,7,8-tetrahydro-4-methoxy-6-methyl-1,3-dioxolo[4,5-g]isoquinolin-5-yl)-, hydrochloride, [S-(R*,S*)]-: Environment tier I assessment
IMAP assessments - 1(3H)-Isobenzofuranone, 6,7-dimethoxy-3-(5,6,7,8-tetrahydro-4-methoxy-6-methyl-1,3-dioxolo[4,5-g]isoquinolin-5-yl)-, hydrochloride, [S-(R*,S*)]-: Human health tier I assessment
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=MFLVZFXCSKVCSH-URBRKQAFSA-N
- Australian Industrial Chemicals Introduction Scheme (AICIS)1(3H)-Isobenzofuranone, 6,7-dimethoxy-3-(5,6,7,8-tetrahydro-4-methoxy-6-methyl-1,3-dioxolo[4,5-g]isoquinolin-5-yl)-, hydrochloride, [S-(R*,S*)]-https://services.industrialchemicals.gov.au/search-assessments/1(3H)-Isobenzofuranone, 6,7-dimethoxy-3-(5,6,7,8-tetrahydro-4-methoxy-6-methyl-1,3-dioxolo[4,5-g]isoquinolin-5-yl)-, hydrochloride, [S-(R*,S*)]-https://services.industrialchemicals.gov.au/search-inventory/
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/Noscapine hydrochloridehttps://commonchemistry.cas.org/detail?cas_rn=912-60-7
- EPA DSSToxNoscapine hydrochloridehttps://comptox.epa.gov/dashboard/DTXSID6073239CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeNoscapine hydrochloridehttps://echa.europa.eu/substance-information/-/substanceinfo/100.011.832Noscapine Hydrochloridehttps://echa.europa.eu/substance-information/-/substanceinfo/100.241.331Noscapine hydrochloride (EC: 213-014-9)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/19631Noscapine Hydrochloride (EC: 811-117-9)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/249017
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingNOSCAPINE HYDROCHLORIDEhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/TTN62ITH9I
- CCSbaseCCSbase Classificationhttps://ccsbase.net/
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.html
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- Springer Nature
- Wikidatanoscapine hydrochloridehttps://www.wikidata.org/wiki/Q27290347
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlAntitussive Agentshttps://www.ncbi.nlm.nih.gov/mesh/68000996
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 388598768https://pubchem.ncbi.nlm.nih.gov/substance/388598768

 CID 275196 (Noscapine)
CID 275196 (Noscapine) CID 313 (Hydrochloric Acid)
CID 313 (Hydrochloric Acid)