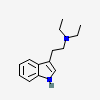
N,N-Diethyltryptamine
PubChem CID
6090
Chemical Safety
Molecular Formula
Synonyms
- N,N-Diethyltryptamine
- Diethyltryptamine
- 61-51-8
- 3-(2-Diethylaminoethyl)indole
- N,N-Diethyl-2-(1H-indol-3-yl)ethanamine
Molecular Weight
216.32 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Dates
- Create:2005-03-27
- Modify:2025-01-18
Description
N,n-diethyltryptamine is a member of tryptamines.
Diethyltryptamine is a DEA Schedule I controlled substance. Substances in the DEA Schedule I have no currently accepted medical use in the United States, a lack of accepted safety for use under medical supervision, and a high potential for abuse. It is a Hallucinogenic substances substance.
Diethyltryptamine (DET) is an orally active hallucinogenic agent and a substituted form of tryptamine.
Chemical Structure Depiction
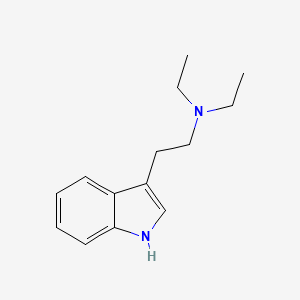
N,N-diethyl-2-(1H-indol-3-yl)ethanamine
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C14H20N2/c1-3-16(4-2)10-9-12-11-15-14-8-6-5-7-13(12)14/h5-8,11,15H,3-4,9-10H2,1-2H3
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
LSSUMOWDTKZHHT-UHFFFAOYSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
CCN(CC)CCC1=CNC2=CC=CC=C21
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C14H20N2
Computed by PubChem 2.2 (PubChem release 2021.10.14)
20671-78-7 (oxalate[1:1])
63938-63-6 (mono-hydrochloride)
102-975-9
7434 (DEA schedule I controlled substance)
- N,N-diethyltryptamine
- N,N-diethyltryptamine monohydrochloride
- N,N-diethyltryptamine oxalate (1:1)
- N,N-Diethyltryptamine
- Diethyltryptamine
- 61-51-8
- 3-(2-Diethylaminoethyl)indole
- N,N-Diethyl-2-(1H-indol-3-yl)ethanamine
- 1H-Indole-3-ethanamine, N,N-diethyl-
- INDOLE, 3-(2-(DIETHYLAMINO)ETHYL)-
- CHEMBL142936
- D.E.T.
- 916E8V4S2V
- BRN 0153320
- UNII-916E8V4S2V
- DEA No. 7434
- NN-Diethyltryptamine
- Oprea1_105894
- 5-22-10-00050 (Beilstein Handbook Reference)
- SCHEMBL517713
- DTXSID9052763
- CHEBI:184081
- LSSUMOWDTKZHHT-UHFFFAOYSA-N
- BDBM50094676
- STK368075
- AKOS005444987
- DB01460
- 3-(2-(DIETHYLAMINO)ETHYL)INDOLE
- Diethyl-[2-(1H-indol-3-yl)-ethyl]-amine
- NS00098694
- N,N-Diethyl-2-(1H-indol-3-yl)ethanamine #
- C22726
- Q2617768
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
216.32 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
XLogP3
Property Value
3.3
Reference
Computed by XLogP3 3.0 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
1
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
1
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
5
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
216.162648646 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
216.162648646 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
19 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
16
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
201
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
1
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
169–171 °C
148.6 Ų [M+H]+ [CCS Type: TW; Method: Major Mix IMS/Tof Calibration Kit (Waters)]
Standard non-polar
1875 , 1900 , 1886.7 , 1910
Semi-standard non-polar
1913.1
Pharmaceuticals -> Listed in ZINC15
S55 | ZINC15PHARMA | Pharmaceuticals from ZINC15 | DOI:10.5281/zenodo.3247749
Pharmaceuticals -> Synthetic Cannabinoids or Psychoactive Compounds
S58 | PSYCHOCANNAB | Synthetic Cannabinoids and Psychoactive Compounds | DOI:10.5281/zenodo.3247723
Pharmaceuticals
S72 | NTUPHTW | Pharmaceutically Active Substances from National Taiwan University | DOI:10.5281/zenodo.3955664
NIST Number
246229
Library
Main library
Total Peaks
88
m/z Top Peak
86
m/z 2nd Highest
130
m/z 3rd Highest
58
Thumbnail
NIST Number
123163
Library
Replicate library
Total Peaks
27
m/z Top Peak
86
m/z 2nd Highest
58
m/z 3rd Highest
87
Thumbnail
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Same Parent, Connectivity Count
Same Parent, Exact Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
PubMed Count
Substance
Diethyltryptamine
DEA Controlled Substances Code Number
7434
Controlled Substances Act Schedule
Schedule I - Substances in the DEA Schedule I have no currently accepted medical use in the United States, a lack of accepted safety for use under medical supervision, and a high potential for abuse.
Class
Hallucinogenic substances
Pharmaceuticals
S72 | NTUPHTW | Pharmaceutically Active Substances from National Taiwan University | DOI:10.5281/zenodo.3955664
Pictogram(s)

Signal
Warning
GHS Hazard Statements
H336 (100%): May cause drowsiness or dizziness [Warning Specific target organ toxicity, single exposure; Narcotic effects]
Precautionary Statement Codes
P261, P271, P304+P340, P319, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
ECHA C&L Notifications Summary
The GHS information provided by 1 company from 1 notification to the ECHA C&L Inventory.
STOT SE 3 (100%)
DEA Controlled Substances
DEA schedule I controlled substance
21 CFR Sections 1308.11-1308.15 https://www.ecfr.gov/current/title-21/chapter-II/part-1308
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=LSSUMOWDTKZHHT-UHFFFAOYSA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/N,N-Diethyltryptaminehttps://commonchemistry.cas.org/detail?cas_rn=61-51-8
- ChemIDplusN,N-Diethyltryptaminehttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000061518ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_useDiethyltryptaminehttps://www.drugbank.ca/drugs/DB01460
- EPA DSSToxN,N-Diethyltryptaminehttps://comptox.epa.gov/dashboard/DTXSID9052763CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeN,N-DIETHYLTRYPTAMINEhttps://echa.europa.euN,N-DIETHYLTRYPTAMINE (EC: 102-975-9)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/389754
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingN,N-DIETHYLTRYPTAMINEhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/916E8V4S2V
- CCSbaseCCSbase Classificationhttps://ccsbase.net/
- ChEBIN,n-diethyltryptaminehttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:184081
- Drug Enforcement Administration (DEA)LICENSEUnless otherwise indicated, information on Department of Justice websites is in the public domain and may be copied and distributed without permission. Citation of the Department of Justice as source of the information is appreciated, as appropriate.https://www.justice.gov/legalpoliciesDiethyltryptaminehttps://www.deadiversion.usdoj.gov/schedules/DEA drug and chemical classificationhttps://www.dea.gov/drug-information/drug-scheduling
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- Therapeutic Target Database (TTD)N,N-diethyl-2-(1H-indol-3-yl)ethanaminehttps://idrblab.net/ttd/data/drug/details/D0W2HW
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.html
- Metabolomics Workbench
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawIndole, 3-(2-(diethylamino)ethyl)-http://www.nist.gov/srd/nist1a.cfm
- SpectraBaseN,N-Diethyltryptaminehttps://spectrabase.com/spectrum/JHf9rpeAdJwN,N-Diethyltryptaminehttps://spectrabase.com/spectrum/JI56j7tcQrY3-[2-(diethylamino)ethyl]indolehttps://spectrabase.com/spectrum/GA2S3XEUK5k3-[2-(DIETHYLAMINO)ETHYL]INDOLEhttps://spectrabase.com/spectrum/I3lGRnNqwTbINDOLE-N,N-DIETHYL-TRYPTAMINEhttps://spectrabase.com/spectrum/DppNnJ84229
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/N,N-DiethyltryptamineNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- PharosLICENSEData accessed from Pharos and TCRD is publicly available from the primary sources listed above. Please respect their individual licenses regarding proper use and redistribution.https://pharos.nih.gov/aboutDiethyl-[2-(1H-indol-3-yl)-ethyl]-aminehttps://pharos.nih.gov/ligands/H96MCJ4U4QJF
- Springer Nature
- WikidataN,N-diethyltryptaminehttps://www.wikidata.org/wiki/Q2617768
- WikipediaChlorpropamidehttps://en.wikipedia.org/wiki/ChlorpropamideDiethyltryptaminehttps://en.wikipedia.org/wiki/Diethyltryptamine
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlN,N-diethyltryptaminehttps://www.ncbi.nlm.nih.gov/mesh/67038927
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 391371218https://pubchem.ncbi.nlm.nih.gov/substance/391371218
- NCBI
CONTENTS