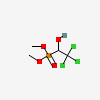
Trichlorfon
- TRICHLORFON
- Metrifonate
- Chlorophos
- 52-68-6
- Metriphonate
- Create:2005-03-26
- Modify:2025-01-18
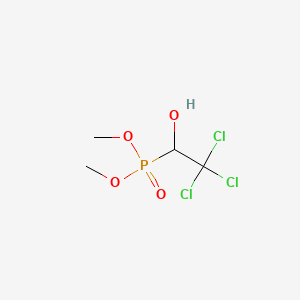
- Bilarcil
- Chlorofos
- Chlorophos
- Dipterex
- Dylox
- Foschlor
- Metrifonate
- Metriphonate
- Neguvon
- Ricifon
- Trichlorfon
- Trichlorphon
- TRICHLORFON
- Metrifonate
- Chlorophos
- 52-68-6
- Metriphonate
- Trichlorphon
- Methyl chlorophos
- Trichlorofon
- Chloroxyphos
- Bilarcil
- Chlorofos
- Chloroftalm
- Dylox
- DETF
- Chlorophthalm
- Fliegenteller
- Trichlorophon
- Agroforotox
- Dipterex
- Diptevur
- Ditrifon
- Foschlor
- Hypodermacid
- Khloroftalm
- Metrifonatum
- Phoschlor
- Polfoschlor
- Ritsifon
- Volfartol
- Anthon
- Bovinox
- Briten
- Briton
- Cekufon
- Combot
- Forotox
- Loisol
- Masoten
- Mazoten
- Neguvon
- Proxol
- Ricifon
- Soldep
- Sotipox
- Trinex
- Votexit
- Wotexit
- Danex
- Dyrex
- Dyvon
- Tugon
- Trichlorphon FN
- (+-)-Trichlorfon
- Equino-Aid
- Foschlor R
- Chlorophosciclosom
- Flibol E
- Bayer L 1359
- DEP (pesticide)
- Foschlorem
- Metrifonato
- Trichloorfon
- Chlorak
- Clorofos
- Denkaphon
- Dimethyl (2,2,2-trichloro-1-hydroxyethyl)phosphonate
- Dimetox
- Dioxaphos
- Dipterex 50
- Phoschlor R50
- Metriphonatum
- Chlorfos
- Chlorphos
- Ciclosom
- Leivasom
- Britten
- Depthon
- Dipterex WP 80
- Foschlor R-50
- Combot equine
- Dicontal Fort
- Tugon fly bait
- Dipterex SL
- Ditriphon 50
- Tugon stable spray
- Foschlor 25
- Aerol 1
- WEC 50
- Trichlorophone
- BAY-a 9826
- Bayer L 13/59
- Dipterax
- Metifonate
- Trichlorfon [USAN]
- Triclorfon
- Satox 20WSC
- Aerol 1 (pesticide)
- Caswell No. 385
- NCI-C54831
- Vermicide bayer 2349
- BAY 15922
- ENT 19,763
- Bayer 15922
- OMS 800
- BAY-L 1359
- 1-Hydroxy-2,2,2-trichloroethylphosphonic acid dimethyl ester
- CCRIS 1289
- HSDB 881
- Neguron
- Onefon
- Phosphonic acid, (2,2,2-trichloro-1-hydroxyethyl)-, dimethyl ester
- UNII-DBF2DG4G2K
- Bayer-L-1359
- DBF2DG4G2K
- Metrifonatum [INN-Latin]
- Metrifonato [INN-Spanish]
- NSC 8923
- NSC-8923
- (+)-Trichlorfon
- (-)-Trichlorfon
- EINECS 200-149-3
- EPA Pesticide Chemical Code 057901
- Foschlor R 50
- Metrifonate [INN]
- Dimethyltrichlorohydroxyethyl phosphonate
- BRN 1709434
- CHEBI:6908
- WEC-50
- Dimethyl 2,2,2-trichloro-1-hydroxyethylphosphonate
- DTXSID0021389
- Metrifonate, (+)-
- Metrifonate, (-)-
- AI3-19763
- Dimethyl (1-hydroxy-2,2,2-trichloroethyl)phosphonate
- O,O-Dimethyl-1-oxy-2,2,2-trichloroethyl phosphonate
- O,O-Dimethyl (1-hydroxy-2,2,2-trichloroethyl)phosphonate
- O,O-Dimethyl (2,2,2-trichloro-1-hydroxyethyl)phosphonate
- (2,2,2-Trichloro-1-hydroxyethyl)phosphonic acid dimethyl ester
- ENT-19,763
- O,O-Dimethyl-(1-hydroxy-2,2,2-trichloraethyl)phosphosaeure ester
- Phosphonic acid, (1-hydroxy-2,2,2-trichloroethyl)-, dimethyl ester
- 1MVY4KU98F
- 37VCQ9C0OG
- Dimethyl 1-hydroxy-2,2,2-trichloroethyl phosphonate
- Dimethyl(2,2,2-trichloro-1-hydroxyethyl)phosphonate
- O,O-Dimethyl (1-oxy-2,2,2-trichloroethyl)phosphonate
- O,O-Dimethyl-(1-hydroxy-2,2,2-trichlorathyl)-phosphat
- O,O-Dimethyl-1-hydroxy-2,2,2-trichloroethylphosphonate
- O,O-Dimetil-(2,2,2-tricloro-1-idrossi-etil)-fosfonato
- MLS000069726
- 1-Hydroxy-2,2,2-trichloro-ethyle phosphonate de dimethyle
- CHEMBL167150
- Dimethoxy-2,2,2-trichloro-1-hydroxy-ethyl phosphine oxide
- Dimethoxy-2,2,2-trichloro-1-hydroxy-ethyl-phosphine oxide
- DTXCID701389
- O,O-Dimethyl (2,2,2-trichloro-1-hydroxyethyl)phosponate
- O,O-Dimethyl-(2,2,2-trichloor-1-hydroxy-ethyl)-fosfonaat
- O,O-Dimethyl-(2,2,2-trichlor-1-hydroxy-aethyl)phosphonat
- 2,2,2-trichloro-1-dimethoxyphosphorylethanol
- (2,2,2-Trichloro-1-hydroxyethyl)-phosphonic acid dimethyl ester
- O,O Dimetil 2,2,2-trichloro 1 hidroxietil fosfonato (Portugese)
- Trichlorfon (USAN)
- NCGC00018237-03
- SMR000058176
- Dimethyl (trichlorohydroxyethyl)phosphonate
- O,O-Dimethyl-(1-hydroxy-2,2,2-trichloraethyl)phosphonsaeure ester
- Metrifonatum (INN-Latin)
- TRICHLORFON (IARC)
- TRICHLORFON [IARC]
- Metrifonato (INN-Spanish)
- METRIFONATE (MART.)
- METRIFONATE [MART.]
- TRICHLORFON (USP-RS)
- TRICHLORFON [USP-RS]
- 26373-97-7
- 56042-27-4
- Dimethyl 1-hydroxy-2,2,2-trichloroethylphosphonate
- Foschlorem [Polish]
- Trichlorphon [ISO]
- Trichloorfon [Dutch]
- Trichlorfon [German]
- METRIFONATE (EP IMPURITY)
- METRIFONATE [EP IMPURITY]
- trichlorphene
- METRIFONATE (USP IMPURITY)
- METRIFONATE [USP IMPURITY]
- METRIFONATE (USP MONOGRAPH)
- METRIFONATE [USP MONOGRAPH]
- DIMETHYL (P)-(2,2,2-TRICHLORO-1-HYDROXYETHYL)PHOSPHONATE
- DIMETHYL (RS)-2,2,2-TRICHLORO-1-HYDROXYETHYLPHOSPHONATE
- Trichlorfon [BSI:ISO]
- Phosphonic acid, (2,2,2-trichloro-1-hydroxyethyl)-, dimethyl ester, (+)-
- Phosphonic acid, (2,2,2-trichloro-1-hydroxyethyl)-, dimethyl ester, (-)-
- Phosphonic acid, P-(2,2,2-trichloro-1-hydroxyethyl)-, dimethyl ester, (+)-
- Phosphonic acid, P-(2,2,2-trichloro-1-hydroxyethyl)-, dimethyl ester, (-)-
- Foschlor R-50 (VAN)
- 56042-26-3
- Clorofos [Russian]
- Chlorophose
- Foschlorine
- Ditrofon
- Ertefon
- Memobay
- Zeltivar
- ProMem
- Metrifonate; Dimethyl (RS)-(2,2,2-trichloro-1-hydroxyethyl)phosphonate; Trichlorfon
- CAS-52-68-6
- Dylox-metasystox-R
- Phoschlor R.50
- Prestwick_599
- Totalene (Salt/Mix)
- (+/-)-Trichlorfon
- EQUINO-ACID
- TRI CHLORFON
- NEGUVON A
- starbld0043468
- Opera_ID_345
- Bayer 2349
- Metrifonate (USP/INN)
- TRICHLORFON [MI]
- 1-Hydroxy-2,2,2-trichloro-ethyle phosphonate de dimethyle [French]
- O,O-Dimethyl-(1-hydroxy-2,2,2-trichlorathyl)-phosphat [German]
- O,O-Dimethyl-(2,2,2-trichloor-1-hydroxy-ethyl)-fosfonaat [Dutch]
- O,O-Dimethyl-(2,2,2-trichlor-1-hydroxy-aethyl)phosphonat [German]
- O,O-Dimetil-(2,2,2-tricloro-1-idrossi-etil)-fosfonato [Italian]
- Prestwick0_000051
- Prestwick1_000051
- Prestwick2_000051
- Prestwick3_000051
- DYLOX-METASYSTOX R
- TRICHLORFON [ISO]
- (.+/-.)-Trichlorfon
- TRICHLORFON [HSDB]
- UNII-1MVY4KU98F
- UNII-37VCQ9C0OG
- O,O-Dimethyl-(1-hydroxy-2,2,2-trichloraethyl)phosphonsaeure ester [German]
- cid_5853
- Bay-a-9826
- Dyrex Bolus, Dyrex Granules
- SCHEMBL15972
- BSPBio_000201
- METRIFONATE [WHO-DD]
- METRIFONATE [WHO-IP]
- SPBio_002122
- WLN: GXGGYQPO&O1&O1
- BPBio1_000223
- O,2,2-trichlorathyl)-phosphat
- O,2,2-trichloroethyl)phosphate
- TRICHLORFON [GREEN BOOK]
- BAYER 13/59
- NSC8923
- P02BB01
- HMS1568K03
- HMS2095K03
- Pharmakon1600-01505765
- HY-B1220
- METRIFONATUM [WHO-IP LATIN]
- P-(2,2,2-Trichloro-1-hydroxyethyl)phosphonic acid dimethyl ester
- Tox21_110843
- Tox21_201607
- Tox21_300860
- BDBM50286920
- MFCD00055272
- NSC759241
- Phosphonic acid, (2,2,2-trichloro-1-hydroxyethyl)-,dimethyl ester
- AKOS001499170
- AKOS022032084
- Tox21_110843_1
- CCG-213546
- CS-4845
- DB11473
- NSC-759241
- O,2,2-trichlorohydroxyethyl)phosphonate
- USEPA/OPP Pesticide Code: 057901
- 2,2,2-Trichloroethyl dimethyl phosphate
- NCGC00018237-02
- NCGC00018237-04
- NCGC00018237-06
- NCGC00018237-07
- NCGC00018237-09
- NCGC00089822-02
- NCGC00089822-03
- NCGC00254763-01
- NCGC00259156-01
- O,2,2-trichloraethyl)phosphosaeure ester
- DA-55441
- O,2,2-tricloro-1-idrossi-etil)-fosfonato
- SBI-0206912.P001
- Trichlorfon 100 microg/mL in Acetonitrile
- 0,2,2-trichloro-1-hydroxyethyl)phosphonate
- O,2,2-trichloro-1-hydroxyethyl)phosphonate
- NS00010188
- O,2,2-trichloor-1-hydroxy-ethyl)-fosfonaat
- O,2,2-trichlor-1-hydroxy-aethyl)phosphonat
- C07971
- D00805
- F21293
- Trichlorfon, PESTANAL(R), analytical standard
- Q422991
- SR-01000000181
- Dimethyl (1-hydroxy-2,2-trichloroethyl)phosphonate
- Dimethyl (2,2,2-trichlorohydroxyethyl) phosphonate
- Dimethyl (2,2-trichloro-1-hydroxyethyl)phosphonate
- Q-201859
- SR-01000000181-2
- (2,2,2-Trichloro-1-hydroxyethyl) dimethylphosphonate
- [(2,2-Trichloro-1-hydroxyethyl) dimethylphosphonate]
- BRD-A22101059-001-13-0
- BRD-A22101059-001-14-8
- O,O-Dimethyl (2,2,2-trichlorohydroxyethyl)phosphonate
- O,O-dimethyl 1-oxy-2,2,2-trichloroethyl phosphonate
- Phosphonic acid,2,2-trichloroethyl)-, dimethyl ester
- trichloro-alpha-hydroxyethyl phosphonic dimethyl ester
- ((2,2,2-Trichloro-1-hydroxyethyl) dimethylphosphonate)
- 1-Hydroxy-2,2-trichloro-ethyle phosphonate de dimethyle
- Dimethoxy-(2,2-trichloro-1-hydroxyethyl)phosphine oxide
- O,O-Dimethyl (1-hydroxy-2,2,2-trichloroethyl)phosphate
- o,o-dimethyl-2,2,2-trichloro-1-hydroxyethyl phosphonate
- (2,2-Trichloro-1-hydroxy)phosphonic acid, dimethyl ester
- (2,2-Trichloro-1-hydroxyethyl)phosphonate, dimethyl ester
- Dimethoxy-(2,2,2-trichloro-1-hydroxyethyl)phosphine oxide
- DIMETHYL-1-HYDROXY-2,2,2-TRICHLOROETHYL PHOSPHONATE
- Metrifonate, European Pharmacopoeia (EP) Reference Standard
- O,O-Dimethyl(2,2,2-tri-chloro-1-hydroxyethyl)phosphonate
- O,O-Dimethyl-(1-hydroxy-2,2,2-trichloro)ethyl phosphate
- O,O-Dimethyl-(2,2,2-trichloro-1-idrossi-etil)-fosfonato
- (1-Hydroxy-2,2-trichloroethyl)phosphonic acid, dimethyl ester
- (2,2,2-Trichloro-1-hydroxyethyl)phosphonate, dimethyl ester
- (2,2-Trichloro-1-hydroxyethyl)phosphonic acid dimethyl ester
- 1-Hydroxy-2,2,2-trichloroethylphosphonate-O,O-dimethyl ester
- DIMETHOXY-2,2,2-TRICHLORO-1-HYDROXYETHYLPHOSPHINE OXIDE
- O,O-dimethyl 1 (2,2,2-trichloro-1-hydroxyethyl)-phosphonate
- PHOSPHONIC ACID, (2,2,2-TRICHLORO-1-HYDROXYETHYL)-,DI
- Phosphonic acid,2,2-trichloro-1-hydroxyethyl)-, dimethyl ester
- (1-Hydroxy-2,2,2-trichloroethyl)phosphonic acid, dimethyl ester
- 2,2,2-TRICHLORO-1-HYDROXYETHYL-PHOSPHONATE, DIMETHYL ESTER
- 2,2-TRICHLORO-1-HYDROXYETHYL)PHOSPHONIC ACID, DIMETHYL ESTER
- O,O-DIMETHYL(1-HYDROXY-2,2,2-TRICHLOROETHYL)PHOSPHONATE
- Trichlorfon, United States Pharmacopeia (USP) Reference Standard
- (2,2,2-TRICHLORO-1-HYDROXYETHYL)PHOSPHONIC ACID, DIMETHYL ESTER
- Phosphonic acid, P-(2,2,2-trichloro-1-hydroxyethyl)-, dimethyl ester
- InChI=1/C4H8Cl3O4P/c1-10-12(9,11-2)3(8)4(5,6)7/h3,8H,1-2H
148.73 Ų [M+H]+ [CCS Type: TW; Method: calibrated with polyalanine and drug standards]
142.65 Ų [M+Na]+ [CCS Type: TW; Method: calibrated with polyalanine and drug standards]
135.96 Ų [M+H]+
148.98 Ų [M+Na]+
109.0 99.99
79.0 61.67
110.0 52.78
145.0 43.77
139.0 33.33
109 99.99
79 61.67
110 52.78
145 43.77
139 33.33
109.0041 999
78.9934 430
82.9438 151
93.0088 56
127.0145 47
46.9674 999
82.9439 337
47.9754 242
109 999
79 617
110 528
145 438
139 333
- Atropine; Trichlorfon (component of)
- Oxfendazole; Trichlorfon (component of)
- Mebendazole; Trichlorfon (component of)
- Thiabendazole; Trichlorfon (component of)
- Phenothiazine; Piperazine Dihydrochloride; Trichlorfon (component of)
- trichlorfon (ISO); dimethyl 2,2,2-trichloro-1-hydroxyethylphosphonate (annotation moved to)
Information on 3 consumer products that contain Trichlorfon in the following categories is provided:
• Pesticides
• Pet Care




H301 (30%): Toxic if swallowed [Danger Acute toxicity, oral]
H302+H312 (47.5%): Harmful if swallowed or in contact with skin [Warning Acute toxicity, oral; acute toxicity, dermal]
H302 (70%): Harmful if swallowed [Warning Acute toxicity, oral]
H312 (47.5%): Harmful in contact with skin [Warning Acute toxicity, dermal]
H317 (100%): May cause an allergic skin reaction [Warning Sensitization, Skin]
H334 (47.5%): May cause allergy or asthma symptoms or breathing difficulties if inhaled [Danger Sensitization, respiratory]
H400 (100%): Very toxic to aquatic life [Warning Hazardous to the aquatic environment, acute hazard]
H410 (100%): Very toxic to aquatic life with long lasting effects [Warning Hazardous to the aquatic environment, long-term hazard]
P233, P260, P261, P264, P270, P271, P272, P273, P280, P284, P301+P316, P301+P317, P302+P352, P304+P340, P317, P321, P330, P333+P317, P342+P316, P362+P364, P391, P403, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 80 reports by companies from 3 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Acute Tox. 3 (30%)
Acute Tox. 4 (70%)
Acute Tox. 4 (47.5%)
Skin Sens. 1 (100%)
Resp. Sens. 1 (47.5%)
Aquatic Acute 1 (100%)
Aquatic Chronic 1 (100%)
Acute toxicity - category 4
Skin sensitisation - category 1
Hazardous to the aquatic environment (acute) - category 1
Hazardous to the aquatic environment (chronic) - category 1
Excerpt from ERG Guide 152 [Substances - Toxic (Combustible)]:
Combustible material: may burn but does not ignite readily. Containers may explode when heated. Those substances designated with a (P) may polymerize explosively when heated or involved in a fire. Runoff may pollute waterways. Substance may be transported in a molten form. (ERG, 2024)
EYES: First check the victim for contact lenses and remove if present. Flush victim's eyes with water or normal saline solution for 20 to 30 minutes while simultaneously calling a hospital or poison control center. Do not put any ointments, oils, or medication in the victim's eyes without specific instructions from a physician. IMMEDIATELY transport the victim after flushing eyes to a hospital even if no symptoms (such as redness or irritation) develop.
SKIN: IMMEDIATELY flood affected skin with water while removing and isolating all contaminated clothing. Gently wash all affected skin areas thoroughly with soap and water. IMMEDIATELY call a hospital or poison control center even if no symptoms (such as redness or irritation) develop. IMMEDIATELY transport the victim to a hospital for treatment after washing the affected areas.
INHALATION: IMMEDIATELY leave the contaminated area; take deep breaths of fresh air. IMMEDIATELY call a physician and be prepared to transport the victim to a hospital even if no symptoms (such as wheezing, coughing, shortness of breath, or burning in the mouth, throat, or chest) develop. Provide proper respiratory protection to rescuers entering an unknown atmosphere. Whenever possible, Self-Contained Breathing Apparatus (SCBA) should be used; if not available, use a level of protection greater than or equal to that advised under Protective Clothing.
INGESTION: DO NOT INDUCE VOMITING. If the victim is conscious and not convulsing, administer a slurry of activated charcoal in water and simultaneously call a hospital or poison control center. IMMEDIATELY transport the victim to a hospital. If the victim is convulsing or unconscious, do not give anything by mouth, ensure that the victim's airway is open and lay the victim on his/her side with the head lower than the body. DO NOT INDUCE VOMITING. IMMEDIATELY transport the victim to a hospital. (NTP, 1992)
Excerpt from ERG Guide 152 [Substances - Toxic (Combustible)]:
SMALL FIRE: Dry chemical, CO2 or water spray.
LARGE FIRE: Water spray, fog or regular foam. If it can be done safely, move undamaged containers away from the area around the fire. Dike runoff from fire control for later disposal. Avoid aiming straight or solid streams directly onto the product.
FIRE INVOLVING TANKS, RAIL TANK CARS OR HIGHWAY TANKS: Fight fire from maximum distance or use unmanned master stream devices or monitor nozzles. Do not get water inside containers. Cool containers with flooding quantities of water until well after fire is out. Withdraw immediately in case of rising sound from venting safety devices or discoloration of tank. ALWAYS stay away from tanks in direct contact with flames. For massive fire, use unmanned master stream devices or monitor nozzles; if this is impossible, withdraw from area and let fire burn. (ERG, 2024)
Excerpt from ERG Guide 152 [Substances - Toxic (Combustible)]:
IMMEDIATE PRECAUTIONARY MEASURE: Isolate spill or leak area in all directions for at least 50 meters (150 feet) for liquids and at least 25 meters (75 feet) for solids.
SPILL: Increase the immediate precautionary measure distance, in the downwind direction, as necessary.
FIRE: If tank, rail tank car or highway tank is involved in a fire, ISOLATE for 800 meters (1/2 mile) in all directions; also, consider initial evacuation for 800 meters (1/2 mile) in all directions. (ERG, 2024)
Excerpt from ERG Guide 152 [Substances - Toxic (Combustible)]:
ELIMINATE all ignition sources (no smoking, flares, sparks or flames) from immediate area. Do not touch damaged containers or spilled material unless wearing appropriate protective clothing. Stop leak if you can do it without risk. Prevent entry into waterways, sewers, basements or confined areas. Cover with plastic sheet to prevent spreading. Absorb or cover with dry earth, sand or other non-combustible material and transfer to containers. DO NOT GET WATER INSIDE CONTAINERS. (ERG, 2024)
2017 Notice of Intended Changes (NIC): These substances, with their corresponding indices, comprise those for which (1) a BEI is proposed for the first time, (2) a change in the Adopted index is proposed, (3) retention as an NIC is proposed, or (4) withdrawal of the Documentation and adopted BEI is proposed. In each case, the proposals should be considered trial indices during the period they are on the NIC. These proposals were ratified by the ACGIH Board of Directors and will remain on the NIC for approximately one year following this ratification. If the Committee neither finds nor receives any substantive data that change its scientific opinion regarding an NIC BEI, the Committee may then approve its recommendation to the ACGIH Board of Directors for adoption. If the Committee finds or receives substantive data that change its scientific opinion regarding an NIC BEI, the Committee may change its recommendation to the ACGIH Board of Directors for the matter to be either retained on or withdrawn from the NIC. Chemical: Cholinesterase Inhibiting Pesticides. /Cholinesterase Inhibiting Pesticides/
Table: Cholinesterase Inhibiting Pesticides
Alcohols and Polyols
Halogenated Organic Compounds
Sulfonates, Phosphonates, and Thiophosphonates, Organic
IMAP assessments - Phosphonic acid, (2,2,2-trichloro-1-hydroxyethyl)-, dimethyl ester: Environment tier I assessment
IMAP assessments - Phosphonic acid, (2,2,2-trichloro-1-hydroxyethyl)-, dimethyl ester: Human health tier I assessment
Volume 30: (1983) Miscellaneous Pesticides
Volume Sup 7: Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42, 1987; 440 pages; ISBN 92-832-1411-0 (out of print)
Neurotoxin - Predominantly motor
Other Poison - Organophosphate
Skin Sensitizer - An agent that can induce an allergic reaction in the skin.
ACGIH Carcinogen - Not Classifiable.
Organophosphates & carbamates, acute poisoning [Category: Acute Poisoning]
Contact dermatitis, allergic [Category: Skin Disease]
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=NFACJZMKEDPNKN-UHFFFAOYSA-N
- Australian Industrial Chemicals Introduction Scheme (AICIS)Phosphonic acid, P-(2,2,2-trichloro-1-hydroxyethyl)-, dimethyl esterhttps://services.industrialchemicals.gov.au/search-assessments/Phosphonic acid, P-(2,2,2-trichloro-1-hydroxyethyl)-, dimethyl esterhttps://services.industrialchemicals.gov.au/search-inventory/
- CAMEO ChemicalsLICENSECAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data.https://cameochemicals.noaa.gov/help/reference/terms_and_conditions.htm?d_f=falseCAMEO Chemical Reactivity Classificationhttps://cameochemicals.noaa.gov/browse/react
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_useMetrifonatehttps://www.drugbank.ca/drugs/DB11473
- DTP/NCILICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuse
- EPA DSSToxCompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- EPA Safe Drinking Water Act (SDWA)
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeTrichlorfon (EC: 200-149-3)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/22001
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingMETRIFONATE, (+)-https://gsrs.ncats.nih.gov/ginas/app/beta/substances/1MVY4KU98FMETRIFONATE, (-)-https://gsrs.ncats.nih.gov/ginas/app/beta/substances/37VCQ9C0OG
- Hazardous Substances Data Bank (HSDB)
- ILO-WHO International Chemical Safety Cards (ICSCs)
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/
- NJDOH RTK Hazardous Substance List
- Occupational Safety and Health Administration (OSHA)LICENSEMaterials created by the federal government are generally part of the public domain and may be used, reproduced and distributed without permission. Therefore, content on this website which is in the public domain may be used without the prior permission of the U.S. Department of Labor (DOL). Warning: Some content - including both images and text - may be the copyrighted property of others and used by the DOL under a license.https://www.dol.gov/general/aboutdol/copyrightTRICHLORPHONhttps://www.osha.gov/chemicaldata/387
- Risk Assessment Information System (RAIS)LICENSEThis work has been sponsored by the U.S. Department of Energy (DOE), Office of Environmental Management, Oak Ridge Operations (ORO) Office through a joint collaboration between United Cleanup Oak Ridge LLC (UCOR), Oak Ridge National Laboratory (ORNL), and The University of Tennessee, Ecology and Evolutionary Biology, The Institute for Environmental Modeling (TIEM). All rights reserved.https://rais.ornl.gov/
- EU Pesticides Database
- Haz-Map, Information on Hazardous Chemicals and Occupational DiseasesLICENSECopyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://haz-map.com/AboutTrichlorfonhttps://haz-map.com/Agents/1141
- ChEBI
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licence
- Toxin and Toxin Target Database (T3DB)LICENSET3DB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (T3DB) and the original publication.http://www.t3db.ca/downloadsTrichlorfonhttp://www.t3db.ca/toxins/T3D3938
- CCSbaseCCSbase Classificationhttps://ccsbase.net/
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/trichlorfonNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jsp
- Consumer Product Information Database (CPID)LICENSECopyright (c) 2024 DeLima Associates. All rights reserved. Unless otherwise indicated, all materials from CPID are copyrighted by DeLima Associates. No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://www.whatsinproducts.com/contents/view/1/6Consumer Products Category Classificationhttps://www.whatsinproducts.com/
- EPA Chemical and Products Database (CPDat)EPA CPDat Classificationhttps://www.epa.gov/chemical-research/chemical-and-products-database-cpdat
- EPA Pesticide Ecotoxicity Database
- USDA Pesticide Data ProgramTrichlorfonhttps://www.ams.usda.gov/datasets/pdp
- Hazardous Chemical Information System (HCIS), Safe Work Australia
- NITE-CMCDimethyl 2,2,2-trichloro-1-hydroxyethylphosphonate; Trichlorfon; DEP - FY2006 (New/original classication)https://www.chem-info.nite.go.jp/chem/english/ghs/06-imcg-0274e.htmlDimethyl 2,2,2-trichloro-1-hydroxyethylphosphonate [tichlorfon or DEP] - FY2015 (Revised classification)https://www.chem-info.nite.go.jp/chem/english/ghs/15-mhlw-0034e.html
- FDA Approved Animal Drug Products (Green Book)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Joint FAO/WHO Expert Committee on Food Additives (JECFA)LICENSEPermission from WHO is not required for the use of WHO materials issued under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Intergovernmental Organization (CC BY-NC-SA 3.0 IGO) licence.https://www.who.int/about/policies/publishing/copyright
- USGS Columbia Environmental Research CenterLICENSEhttps://www.usgs.gov/foia
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingTrichlorfonhttp://www.hmdb.ca/metabolites/HMDB0254686HMDB0254686_cms_29752https://hmdb.ca/metabolites/HMDB0254686#spectra
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawMetrifonatehttp://www.nist.gov/srd/nist1a.cfm
- SpectraBaseTrichlorfonhttps://spectrabase.com/spectrum/KFSM5Mglq0gNFACJZMKEDPNKN-UHFFFAOYSA-Nhttps://spectrabase.com/spectrum/8HSGsRs6Go6DIMETHYL 1-HYDROXY-2,2,2-TRICHLOROETHYLPHOSPHONATEhttps://spectrabase.com/spectrum/GB5bAbVs2ts
- International Agency for Research on Cancer (IARC)LICENSEMaterials made available by IARC/WHO enjoy copyright protection under the Berne Convention for the Protection of Literature and Artistic Works, under other international conventions, and under national laws on copyright and neighbouring rights. IARC exercises copyright over its Materials to make sure that they are used in accordance with the Agency's principles. All rights are reserved.https://publications.iarc.fr/Terms-Of-UseIARC Classificationhttps://www.iarc.fr/
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlAnatomical Therapeutic Chemical (ATC) classificationhttp://www.genome.jp/kegg-bin/get_htext?br08303.kegAntiinfectiveshttp://www.genome.jp/kegg-bin/get_htext?br08307.kegRisk category of Japanese OTC drugshttp://www.genome.jp/kegg-bin/get_htext?br08312.kegClassification of Japanese OTC drugshttp://www.genome.jp/kegg-bin/get_htext?br08313.kegAnimal drugs in Japanhttp://www.genome.jp/kegg-bin/get_htext?br08331.keg
- MassBank Europe
- Metabolomics Workbench
- NMRShiftDB
- Springer Nature
- SpringerMaterialsdimethyl (2,2,2-trichloro-1-hydroxyethyl)phosphonatehttps://materials.springer.com/substanceprofile/docs/smsid_ukuhffokjxlaviqm
- WHO Anatomical Therapeutic Chemical (ATC) ClassificationLICENSEUse of all or parts of the material requires reference to the WHO Collaborating Centre for Drug Statistics Methodology. Copying and distribution for commercial purposes is not allowed. Changing or manipulating the material is not allowed.https://www.whocc.no/copyright_disclaimer/
- Wikidatametrifonatehttps://www.wikidata.org/wiki/Q422991
- WikipediaAluminium dodecaboridehttps://en.wikipedia.org/wiki/Aluminium_dodecaborideMetrifonatehttps://en.wikipedia.org/wiki/Metrifonate
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlTrichlorfonhttps://www.ncbi.nlm.nih.gov/mesh/68014236Cholinesterase Inhibitorshttps://www.ncbi.nlm.nih.gov/mesh/68002800Anthelminticshttps://www.ncbi.nlm.nih.gov/mesh/68000871Insecticideshttps://www.ncbi.nlm.nih.gov/mesh/68007306
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403402054https://pubchem.ncbi.nlm.nih.gov/substance/403402054
- NCBI