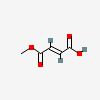
Monomethyl Fumarate
- Monomethyl fumarate
- 2756-87-8
- Fumaric acid monomethyl ester
- mono-Methyl fumarate
- Methyl hydrogen fumarate
- Create:2005-03-26
- Modify:2025-01-04
 Fumaric Acid (has active moiety);
Fumaric Acid (has active moiety);  Dimethyl Fumarate (active moiety of);
Dimethyl Fumarate (active moiety of);  Diroximel Fumarate (active moiety of).
Diroximel Fumarate (active moiety of).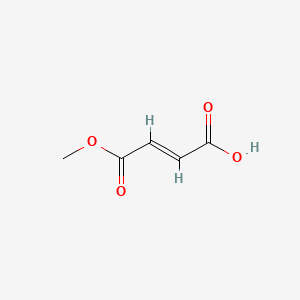
- bafiertam
- methyl hydrogen fumarate
- methylhydrogenfumarate
- monomethyl fumarate
- Monomethyl fumarate
- 2756-87-8
- Fumaric acid monomethyl ester
- mono-Methyl fumarate
- Methyl hydrogen fumarate
- 4-methoxy-4-oxobut-2-enoic acid
- (E)-4-methoxy-4-oxobut-2-enoic acid
- Bafiertam
- (2E)-4-Methoxy-4-oxobut-2-enoic acid
- UNII-45IUB1PX8R
- 2-Butenedioic acid (2E)-, 1-methyl ester
- 45IUB1PX8R
- monomethylis fumaras
- methylhydrogenfumarate
- fumarato de monometilo
- fumarate de monomethyle
- EINECS 220-412-6
- NSC-523835
- Monomethyl fumarate [USAN]
- CHEMBL589586
- CHEBI:167450
- DTXSID801016498
- NSC 523835
- Monomethyl fumarate (USAN)
- MFCD00063174
- 44836-34-2
- monomethyl-fumarate
- Bafiertam (TN)
- (2Z)-2-ButenedioicAcid1-MethylEster-d3
- (2E)-3-(methoxycarbonyl)prop-2-enoic acid
- Monomethylfumarate (MMF)
- mono-Methyl fumarate, 97%
- SCHEMBL60132
- SCHEMBL60133
- Maleic acid mono(methyl ester)
- GTPL5786
- FumarsA currencyuremonomethylester
- DTXCID30209463
- MONOMETHYL FUMARATE [INN]
- BCP18454
- BDBM50342426
- MONOMETHYL FUMARATE [WHO-DD]
- s6889
- WHO 11163
- (E)-2-Butendioic acid, methyl ester
- (E)-4-methoxy-4-oxobut-2-enoicacid
- AKOS015892642
- DB14219
- MRF-0000756
- MONOMETHYL FUMARATE [ORANGE BOOK]
- AS-15353
- DA-55627
- (E)-But-2-enedioic acid monomethyl ester
- FUMARIC ACID MONOMETHYL ESTER [MI]
- (2E)-4-Methoxy-4-oxo-2-butenoic acid #
- HY-103252
- CS-0026484
- M2413
- NS00083454
- A11518
- D11492
- EN300-1699172
- J-017996
- J-522708
- Q27087639
- Fumaric acid monomethyl ester, Methyl hydrogen fumarate
- Z2315585555
- mono-Methyl fumarate, certified reference material, TraceCERT(R)
- UR9
Fumaric Acid (has active moiety)
Dimethyl Fumarate (active moiety of)
Diroximel Fumarate (active moiety of)

P264+P265, P280, P305+P354+P338, and P317
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 140 reports by companies from 6 notifications to the ECHA C&L Inventory.
Reported as not meeting GHS hazard criteria per 37 of 140 reports by companies. For more detailed information, please visit ECHA C&L website.
There are 5 notifications provided by 103 of 140 reports by companies with hazard statement code(s).
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=NKHAVTQWNUWKEO-NSCUHMNNSA-N
- Australian Industrial Chemicals Introduction Scheme (AICIS)2-Butenedioic acid, monomethyl esterhttps://services.industrialchemicals.gov.au/search-inventory/
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/Monomethyl fumaratehttps://commonchemistry.cas.org/detail?cas_rn=2756-87-8
- ChemIDplusMonomethyl fumarate [USAN]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0002756878ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_useMonomethyl fumaratehttps://www.drugbank.ca/drugs/DB14219
- EPA DSSToxMonomethyl fumaratehttps://comptox.epa.gov/dashboard/DTXSID801016498CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeMethyl hydrogen fumaratehttps://chem.echa.europa.eu/100.018.557Methyl hydrogen fumarate (EC: 220-412-6)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/105173
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingMonomethyl Fumaratehttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/45IUB1PX8R
- ChEBIMonomethyl fumaratehttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:167450
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/Monomethyl fumaratehttps://www.wikidata.org/wiki/Q27087639LOTUS Treehttps://lotus.naturalproducts.net/
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licenceMONOMETHYL FUMARATEhttps://platform.opentargets.org/drug/CHEMBL589586
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- Drug Gene Interaction database (DGIdb)LICENSEThe data used in DGIdb is all open access and where possible made available as raw data dumps in the downloads section.http://www.dgidb.org/downloadsMONOMETHYL FUMARATEhttps://www.dgidb.org/drugs/rxcui:1546433
- IUPHAR/BPS Guide to PHARMACOLOGYLICENSEThe Guide to PHARMACOLOGY database is licensed under the Open Data Commons Open Database License (ODbL) https://opendatacommons.org/licenses/odbl/. Its contents are licensed under a Creative Commons Attribution-ShareAlike 4.0 International License (http://creativecommons.org/licenses/by-sa/4.0/)https://www.guidetopharmacology.org/about.jsp#licensemonomethyl fumaratehttps://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5786Guide to Pharmacology Target Classificationhttps://www.guidetopharmacology.org/targets.jsp
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- DailyMed
- Drugs@FDALICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingMONOMETHYL FUMARATEhttps://www.accessdata.fda.gov/scripts/cder/daf/
- FDA Orange BookLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlUSP drug classificationhttp://www.genome.jp/kegg-bin/get_htext?br08302.keg
- KNApSAcK Species-Metabolite Database
- Natural Product Activity and Species Source (NPASS)Methyl Fumaratehttps://bidd.group/NPASS/compound.php?compoundID=NPC298413
- Metabolomics WorkbenchMethyl hydrogen fumaratehttps://www.metabolomicsworkbench.org/data/StructureData.php?RegNo=46216
- National Drug Code (NDC) DirectoryLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-law2-Butenedioic acid (E)-, monomethyl esterhttp://www.nist.gov/srd/nist1a.cfm
- SpectraBaseMALEIC ACID, MONOMETHYL ESTERhttps://spectrabase.com/spectrum/2fZPEdwXOCpFUMARIC ACID, MONOMETHYL ESTERhttps://spectrabase.com/spectrum/CrkOkvYlYZEFUMARIC ACID, MONOMETHYL ESTERhttps://spectrabase.com/spectrum/K8SBHsA6INk(E)-BUT-2-ENEDIOIC_ACID_MONOMETHYLESTERhttps://spectrabase.com/spectrum/4kHxhhL1e8Sfumaric acid, monomethyl esterhttps://spectrabase.com/spectrum/5Xh4JQLU6LrFUMARIC ACID, MONOMETHYL ESTERhttps://spectrabase.com/spectrum/CrkBBzzMdYe2-Butenedioic acid, (E)-, monomethyl esterhttps://spectrabase.com/spectrum/IxCrgIwSWghfumaric acid, monomethyl esterhttps://spectrabase.com/spectrum/BoIXlJQuVqf
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.htmlmonomethyl fumaratehttps://rxnav.nlm.nih.gov/id/rxnorm/1546433
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/Monomethylfumarate (MMF)NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- PharosLICENSEData accessed from Pharos and TCRD is publicly available from the primary sources listed above. Please respect their individual licenses regarding proper use and redistribution.https://pharos.nih.gov/aboutmonomethyl fumaratehttps://pharos.nih.gov/ligands/1UAZQW1QGA4J
- Protein Data Bank in Europe (PDBe)
- RCSB Protein Data Bank (RCSB PDB)LICENSEData files contained in the PDB archive (ftp://ftp.wwpdb.org) are free of all copyright restrictions and made fully and freely available for both non-commercial and commercial use. Users of the data should attribute the original authors of that structural data.https://www.rcsb.org/pages/policies
- Springer Nature
- SpringerMaterialsFumarsäuremonomethylesterhttps://materials.springer.com/substanceprofile/docs/smsid_igbfedoudjnnkwss
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidatamonomethyl fumaratehttps://www.wikidata.org/wiki/Q27087639
- WikipediaPrenylated flavin mononucleotidehttps://en.wikipedia.org/wiki/Prenylated_flavin_mononucleotideMonomethyl fumaratehttps://en.wikipedia.org/wiki/Monomethyl_fumarate
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlmonomethyl fumaratehttps://www.ncbi.nlm.nih.gov/mesh/67509058
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403388495https://pubchem.ncbi.nlm.nih.gov/substance/403388495
- NCBI