Lysine orotate
PubChem CID
119786
Chemical Safety
Molecular Formula
- C6H14N2O2.C5H4N2O4
- C11H18N4O6
Synonyms
- Lysine orotate
- L-Lysine orotate
- 28003-86-3
- Lysortine
- Hepa-Galen
Molecular Weight
302.28 g/mol
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Component Compounds
Dates
- Create:2005-08-08
- Modify:2025-01-04
Chemical Structure Depiction
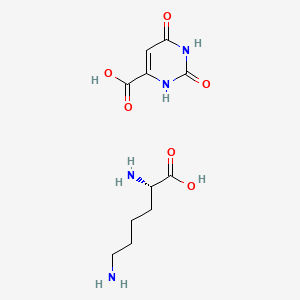
Conformer generation is disallowed since mixture or salt
SVG Image

(2S)-2,6-diaminohexanoic acid;2,4-dioxo-1H-pyrimidine-6-carboxylic acid
Computed by Lexichem TK 2.7.0 (PubChem release 2021.10.14)
InChI=1S/C6H14N2O2.C5H4N2O4/c7-4-2-1-3-5(8)6(9)10;8-3-1-2(4(9)10)6-5(11)7-3/h5H,1-4,7-8H2,(H,9,10);1H,(H,9,10)(H2,6,7,8,11)/t5-;/m0./s1
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
FVGJURXBWORGKL-JEDNCBNOSA-N
Computed by InChI 1.0.6 (PubChem release 2021.10.14)
C1=C(NC(=O)NC1=O)C(=O)O.C(CCN)C[C@@H](C(=O)O)N
Computed by OEChem 2.3.0 (PubChem release 2024.12.12)
C6H14N2O2.C5H4N2O4
C11H18N4O6
Computed by PubChem 2.2 (PubChem release 2021.10.14)
28003-86-3
- Lysine orotate
- L-Lysine orotate
- 28003-86-3
- Lysortine
- Hepa-Galen
- L-Lysine mono(1,2,3,6-tetrahydro-2,6-dioxopyrimidine-4-carboxylate)
- L-Lysine (orotate)
- 782D931P40
- (2S)-2,6-diaminohexanoic acid;2,4-dioxo-1H-pyrimidine-6-carboxylic acid
- Orotate de lysine
- Orotate de lysine [French]
- EINECS 248-771-4
- Lysine, monoorotate, L-
- UNII-782D931P40
- SCHEMBL1191152
- LYSINE OROTATE [WHO-DD]
- DTXSID80950690
- FVGJURXBWORGKL-JEDNCBNOSA-N
- AKOS024342579
- DA-65025
- HY-149157
- CS-0646573
- NS00083871
- Q27266631
- 2,6-Dihydroxypyrimidine-4-carboxylic acid--lysine (1/1)
- L-LYSINE, 1,2,3,6-TETRAHYDRO-2,6-DIOXO-4-PYRIMIDINECARBOXYLATE (1:1)
- 2,6-dioxo-1,2,3,6-tetrahydro-4-pyrimidinecarboxylic acid compound with (2S)-2,6-diaminohexanoic acid (1:1)
Property Name
Property Value
Reference
Property Name
Molecular Weight
Property Value
302.28 g/mol
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Donor Count
Property Value
6
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Hydrogen Bond Acceptor Count
Property Value
8
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Rotatable Bond Count
Property Value
6
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Exact Mass
Property Value
302.12263431 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Monoisotopic Mass
Property Value
302.12263431 Da
Reference
Computed by PubChem 2.2 (PubChem release 2021.10.14)
Property Name
Topological Polar Surface Area
Property Value
185 Ų
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Heavy Atom Count
Property Value
21
Reference
Computed by PubChem
Property Name
Formal Charge
Property Value
0
Reference
Computed by PubChem
Property Name
Complexity
Property Value
374
Reference
Computed by Cactvs 3.4.8.18 (PubChem release 2021.10.14)
Property Name
Isotope Atom Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Atom Stereocenter Count
Property Value
1
Reference
Computed by PubChem
Property Name
Undefined Atom Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Defined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Undefined Bond Stereocenter Count
Property Value
0
Reference
Computed by PubChem
Property Name
Covalently-Bonded Unit Count
Property Value
2
Reference
Computed by PubChem
Property Name
Compound Is Canonicalized
Property Value
Yes
Reference
Computed by PubChem (release 2021.10.14)
Follow these links to do a live 2D search or do a live 3D search for this compound, sorted by annotation score. This section is deprecated (see here for details), but these live search links provide equivalent functionality to the table that was previously shown here.
Same Connectivity Count
Mixtures, Components, and Neutralized Forms Count
Similar Compounds (2D)
Similar Conformers (3D)
Same Count
Pictogram(s)

Signal
Warning
GHS Hazard Statements
H302 (100%): Harmful if swallowed [Warning Acute toxicity, oral]
Precautionary Statement Codes
P264, P270, P301+P317, P330, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
ECHA C&L Notifications Summary
The GHS information provided by 1 company from 1 notification to the ECHA C&L Inventory.
Acute Tox. 4 (100%)
The Australian Inventory of Industrial Chemicals
Chemical: L-Lysine, mono(1,2,3,6-tetrahydro-2,6-dioxo-4-pyrimidinecarboxylate)
Chemical Assessment
Evaluation - Chemicals not considered for in depth evaluation - Not commercially active in Australia
- Australian Industrial Chemicals Introduction Scheme (AICIS)L-Lysine, mono(1,2,3,6-tetrahydro-2,6-dioxo-4-pyrimidinecarboxylate)https://services.industrialchemicals.gov.au/search-assessments/L-Lysine, mono(1,2,3,6-tetrahydro-2,6-dioxo-4-pyrimidinecarboxylate)https://services.industrialchemicals.gov.au/search-inventory/
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/L-Lysine orotatehttps://commonchemistry.cas.org/detail?cas_rn=28003-86-3
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSTox2,6-Dihydroxypyrimidine-4-carboxylic acid--lysine (1/1)https://comptox.epa.gov/dashboard/DTXSID80950690CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeL-lysine mono(1,2,3,6-tetrahydro-2,6-dioxopyrimidine-4-carboxylate)https://echa.europa.eu/substance-information/-/substanceinfo/100.044.323L-lysine mono(1,2,3,6-tetrahydro-2,6-dioxopyrimidine-4-carboxylate) (EC: 248-771-4)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/44123
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Wikidatalysine orotatehttps://www.wikidata.org/wiki/Q27266631
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
CONTENTS
 CID 5962 (Lysine)
CID 5962 (Lysine) CID 967 (Orotic Acid)
CID 967 (Orotic Acid)