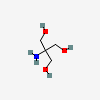
Tromethamine
- Trometamol
- TROMETHAMINE
- 77-86-1
- Tris(Hydroxymethyl)aminomethane
- Tris
- Create:2005-03-26
- Modify:2025-01-18
 Tromethamine hydrochloride (is active moiety of); Potassium chloride; sodium chloride; tromethamine (component of) ... View More ...
Tromethamine hydrochloride (is active moiety of); Potassium chloride; sodium chloride; tromethamine (component of) ... View More ...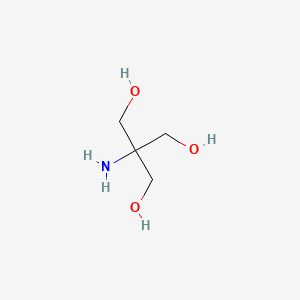
- Tri(hydroxymethyl)aminomethane
- Tris Buffer
- Tris(hydroxymethyl)aminomethane
- Tris-Magnesium(II)-Potassium Chloride Buffer
- Tris-Mg(II)-KCl Buffer
- Trisamine
- Trizma
- Trometamol
- Tromethamine
- Trometamol
- TROMETHAMINE
- 77-86-1
- Tris(Hydroxymethyl)aminomethane
- Tris
- Tham
- 2-Amino-2-(hydroxymethyl)propane-1,3-diol
- Trisamine
- Trizma
- 2-Amino-2-(hydroxymethyl)-1,3-propanediol
- Tris buffer
- Tris base
- Tromethane
- Tris-base
- Trisaminol
- Pehanorm
- Talatrol
- Trisamin
- Trispuffer
- Tutofusin tris
- Addex-tham
- Tris-steril
- 1,3-Propanediol, 2-amino-2-(hydroxymethyl)-
- Tris, free base
- Trimethylolaminomethane
- Aminotrimethylolmethane
- Aminotris(hydroxymethyl)methane
- Tromethanmin
- Tris (buffering agent)
- Tris Amino
- Tris(hydroxymethyl)methanamine
- Tris(hydroxymethyl)methylamine
- THAM-E
- Tromethamolum
- Trometamole
- Trizma base
- Trometamolum
- 2-Amino-2-methylol-1,3-propanediol
- Caswell No. 036
- Tris-hydroxymethylaminomethane
- Tri(hydroxymethyl)aminomethane
- bakerbond(tm) cyano (cn)
- NSC 6365
- Methylamine, 1,1,1-tris(hydroxymethyl)-
- 2-(Hydroxymethyl)-2-amino-1,3-propanediol
- ABX (ANTIBODY EXCHANGER)
- HSDB 3408
- Tris-Amino
- Methanamine, 1,1,1-tris(hydroxymethyl)-
- Trometamolum [INN-Latin]
- NSC-6365
- EINECS 201-064-4
- UNII-023C2WHX2V
- MFCD00004679
- Trometamol [INN]
- Tris-hydroxymethyl-aminomethan
- EPA Pesticide Chemical Code 083901
- 023C2WHX2V
- CHEBI:9754
- WP QUAT (STRONG ANION EXCHANGER)
- 2-Amino-2-hydroxymethyl-1,3-propanediol
- DTXSID2023723
- Tris(hydroxymethyly)amino methane
- AI3-03948
- NSC6365
- 126850-03-1
- 126850-06-4
- 126850-08-6
- Tromethamine [USAN:USP]
- AMINO (NH2) NARROW-PORE MEDIA-NORMAL PHASE
- DTXCID503723
- tris(hydroxymethyl) aminomethane
- 126850-05-3
- Tris-(hydroxymethyl)-aminomethane
- Trometamol(Tris),Proteomics Grade
- EC 201-064-4
- NSC65434
- THAM-E COMPONENT TROMETHAMINE
- 1,1,1-tris(hydroxymethyl)methanamine
- Trometamol(Tris),for molecular biology
- NCGC00159412-02
- NCGC00159412-04
- Tris buffertris-hydroxymethyl-aminomethan
- TROMETHAMINE (II)
- TROMETHAMINE [II]
- Trometamolum (INN-Latin)
- Tromethamine (USAN:USP)
- TROMETAMOL (MART.)
- TROMETAMOL [MART.]
- WLN: Q1XZ1Q1Q
- 136760-04-8
- Trometamol(Tris), inverted exclamation markY99.5%
- TROMETHAMINE (USP-RS)
- TROMETHAMINE [USP-RS]
- 4-Anilino-1-(2-hydroxyethylamino)anthracene-9,11-dione
- Tromethamine [USAN]
- (tris(hydroxymethyl)aminomethane)
- [tris(hydroxymethyl)aminomethane]
- TROMETAMOL (EP MONOGRAPH)
- TROMETAMOL [EP MONOGRAPH]
- 1, 2-amino-2-(hydroxymethyl)-
- Triladyl
- Trigmo base
- TROMETHAMINE (USP MONOGRAPH)
- TROMETHAMINE [USP MONOGRAPH]
- MFCD00132476
- TRIS(HYDROXY-D-METHYL)AMINO-D2-METHANE, 98 ATOM % D
- Methanamine,1,1-tris(hydroxymethyl)-
- Methylamine,1,1-tris(hydroxymethyl)-
- tris(hydroxymethyl)amino methane
- .beta.-D-ribo-Hexopyranose, 1,6-anhydro-3-deoxy-2-O-(1-methylethyl)-4-O-(phenylmethyl)-
- CAS-77-86-1
- Tris(hydroxymethyl)aminomethane, >=99.8%
- propane, 2-amino-1,3-dihydroxy-2-hydroxymethyl-
- Tris(hydroxymethyl)aminomethane, Electrophoresis Grade
- Tris-hydroxymethyl-aminomethan [German]
- trometamolo
- Tromethamin
- Aminotri(hydroxymethyl)methane
- tris-buffer
- tris-amine
- Tro.meta.mole
- Tris(Hydroxymethyl)-Aminomethane
- Tro.meta.mol
- 1gng
- tris base solution
- 83147-39-1
- THAM (Standard)
- TRS
- Tromethamine (USP)
- TRIS Ultrapure, EP
- 1h4n
- Trometamol (JAN/INN)
- TROMETAMOL [JAN]
- TROMETHAMINE [MI]
- SCHEMBL975
- trishydroxymethylmethylamine
- THAM (TN)
- TROMETHAMINE [HSDB]
- NCIOpen2_000263
- NCIOpen2_001720
- TROMETAMOL [WHO-DD]
- TROMETHAMINE [VANDF]
- Oprea1_677781
- trishydroxymethyl aminomethane
- tris-hydroxymethyl-methylamine
- MLS000028643
- tris hydroxymethyl aminomethane
- tris-(hydroxymethyl)methylamine
- Tris (hydroxymethyl)aminoethane
- GTPL7328
- tris (hydroxymethyl)aminomethane
- CHEMBL1200391
- tris (hydroxymethyl) methylamine
- HY-D0227C
- HY-D0227D
- HY-D0227E
- HY-D0227R
- tris (hydroxymethyl) aminomethane
- B05BB03
- B05XX02
- 2-Amino-2-hydroxymethylpropanediol
- HMS3652L05
- HMS3885H09
- tris-(hydroxymethyl)-amino-methane
- TROMETHAMINE [ORANGE BOOK]
- 2-amino-2-hydroxymethyl-propane-1
- Tris-Base, molecular biology grade
- BCP05578
- HY-D0227
- STR03166
- Tox21_111645
- Tox21_201646
- Tox21_303167
- BBL000011
- NSC-65434
- s4176
- STK379529
- 1,1,1-tris(hydroxymethyl)methylamine
- 2-amino-2-methylol-propane-1,3-diol
- AKOS000121321
- Tox21_111645_1
- 1,1,1-Tris(hydroxymethyl)-methylamine
- CCG-214012
- CS-W018524
- DB03754
- PB47623
- Trizma(R) base, >=99.0% (T)
- Tris(hydroxymethyl)aminomethane, >=99%
- TROMETHAMINE COMPONENT OF THAM-E
- NCGC00159412-03
- NCGC00159412-05
- NCGC00257164-01
- NCGC00259195-01
- BP-13394
- DA-58763
- SMR000059179
- Tris(hydroxymethyl)aminomethane ACS grade
- Tris(hydroxymethyl)aminomethane, ultrapure
- 2-amino-2-[hydroxymethyl]-1,3-propandiol
- DB-091324
- DS-014869
- Methanamine, 1, 1,1-tris(hydroxymethyl)-
- Tris acidimetric, NIST(R) SRM(R) 723e
- A0321
- CS-0201542
- CS-0201543
- CS-0201544
- NS00002396
- SW219208-1
- T2516
- TRIS-buffered saline (TBS, 10X) pH 7.4
- TRIS-buffered saline (TBS, 10X) pH 7.6
- TRIS-buffered saline (TBS, 10X) pH 8.0
- TRIS-buffered saline (TBS, 20X) pH 7.4
- 2-(Hydroxymethyl)-2-amino-1, 3-propanediol
- 2-amino-2-(hydroxyl-methyl)propane-1,3-diol
- 2-amino-2-(hydroxymethyl) propane-1,3-diol
- 2-Amino-2-(hydroxymethyl)-1,3-propanediol;
- 2-Amino-2-(hydroxymethyl)-propane-1,3-diol
- EN300-21687
- Trizma(R) base, BioUltra, >=99.8% (T)
- Trizma(R) base, tested according to Ph.Eur.
- Trometamol (part of FOSFOMYCIN TROMETAMOL)
- Tromethamine, meets USP testing specifications
- C07182
- D00396
- M02623
- P17498
- SBI-0653855.0001
- AB00443859_03
- AB00443859_04
- Q413961
- Sigma 7-9(R), >=99% (titration), crystalline
- SR-01000944234
- Trizma(R) base, puriss. p.a., >=99.7% (T)
- J-610076
- SR-01000944234-1
- Trizma(R) base, >=99.9% (titration), crystalline
- Trizma(R) base, Vetec(TM) reagent grade, >=99%
- W-104296
- BRD-K47978074-001-02-4
- BRD-K47978074-001-03-2
- Tris(hydroxymethyl)aminomethane, ACS reagent, 99.9%
- F0001-1979
- Tris(hydroxymethyl)aminomethane, ACS reagent, >=99.8%
- Tris(hydroxymethyl)aminomethane, Molecular Biology Grade
- TRIS-buffered saline (TBS, 10X, high salt) pH 7.4
- Z104509094
- Tris(hydroxymethyl)aminomethane, p.a., ACS reagent, 99.8%
- Tris(hydroxymethyl)aminomethane, ultrapure grade, >=99.9%
- Trizma(R) base, puriss. p.a., buffer substance, >=99.5%
- Trometamol, European Pharmacopoeia (EP) Reference Standard
- Tris(hydroxymethyl)aminomethane, JIS special grade, >=99.0%
- TRIS-buffered saline (TBS, 10X) pH 7.4, for Western blot
- InChI=1/C4H11NO3/c5-4(1-6,2-7)3-8/h6-8H,1-3,5H
- Trizma(R) base, anhydrous, free-flowing, Redi-Dri(TM), >=99.9%
- Trizma(R) base, BioUltra, for molecular biology, >=99.8% (T)
- Tromethamine, United States Pharmacopeia (USP) Reference Standard
- Trizma(R) base, cell culture tested, >=99.9% (titration), crystalline
- Trizma(R) base, BioXtra, pH 10.5-12.0 (1 M in H2O), >=99.9% (titration)
- Trizma(R) base, Primary Standard and Buffer, >=99.9% (titration), crystalline
- Tromethamine, Pharmaceutical Secondary Standard; Certified Reference Material
- 79261-03-3
- Trizma(R) base, BioPerformance Certified, meets EP, USP testing specifications, cell culture tested, >=99.9% (titration)
- Trizma(R) base, certified reference material for titrimetry, certified by BAM, according to ISO 17025, >=99.5%
122.2 100
104.1 16.34
104.9 5.18
50.0 1.13
87.3 0.58
104.1 100
122.2 54.35
56.1 51.88
57.0 35.81
105.2 19.74
122.2 999
104.1 163
104.9 52
50 11
87.3 6
104.1 999
122.2 543
56.1 518
57 358
105.2 197
Tromethamine hydrochloride (is active moiety of)
- Potassium chloride; sodium chloride; tromethamine (component of)
- Ascorbic acid; ascorbyl palmitate; avobenzone; bemotrizinol; butylated hydroxytoluene; butylene glycol; C20-22 alcohols; carbomer copolymer type A (allyl pentaerythritol crosslinked); carbomer homopolymer, unspecified type; chlorphenesin; dimethicone; fragrance 13576; glycerin; linoleic acid; linolenic acid; octinoxate; octisalate; octocrylene; phenoxyethanol; poly(methyl methacrylate; 450000 MW); polyethylene glycol 400; propylene glycol; silicon dioxide; titanium dioxide; tocopherol; tromethamine; water (component of)
- alpha-TOCOPHEROL; ANHYDROUS CITRIC ACID; ASCORBIC ACID; ASCORBYL PALMITATE; AVOBENZONE; BEMOTRIZINOL; BUTYLATED HYDROXYTOLUENE; BUTYLENE GLYCOL; C20-22 ALCOHOLS; CARBOMER COPOLYMER TYPE A (ALLYL PENTAERYTHRITOL CROSSLINKED); CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE; CHLORPHENESIN; DIMETHICONE; FRAGRANCE 13576; GLYCERIN; LINOLEIC ACID; LINOLENIC ACID; OCTINOXATE; OCTISALATE; OCTOCRYLENE; PHENOXYETHANOL; POLY(METHYL METHACRYLATE; 450000 MW); POLYETHYLENE GLYCOL 400; PROPYLENE GLYCOL; SILICON DIOXIDE; TITANIUM DIOXIDE; TROMETHAMINE; WATER (component of)
Leather Tanning and Processing [Category: Industry]
Textiles (Printing, Dyeing, or Finishing) [Category: Industry]
Use (kg; approx.) in Germany (2009): >500
Consumption (g per capita; approx.) in Germany (2009): 0.00611
Calculated removal (%): 92.1
- Laboratory chemicals
- Other (specify)
- Intermediates
- Processing aids, not otherwise listed
- Laboratory chemicals
Information on 2 consumer products that contain Tromethamine in the following categories is provided:
• Personal Care
2019: 1,000,000 lb - <20,000,000 lb
2018: 1,000,000 lb - <20,000,000 lb
2017: 1,000,000 lb - <20,000,000 lb
2016: 1,000,000 lb - <20,000,000 lb
- All Other Basic Organic Chemical Manufacturing
- All Other Chemical Product and Preparation Manufacturing
- Pharmaceutical and Medicine Manufacturing

H315 (81.5%): Causes skin irritation [Warning Skin corrosion/irritation]
H319 (81.6%): Causes serious eye irritation [Warning Serious eye damage/eye irritation]
H335 (71.9%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation]
P261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 1174 reports by companies from 31 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Reported as not meeting GHS hazard criteria per 216 of 1174 reports by companies. For more detailed information, please visit ECHA C&L website.
There are 28 notifications provided by 958 of 1174 reports by companies with hazard statement code(s).
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Skin Irrit. 2 (81.5%)
Eye Irrit. 2 (81.6%)
STOT SE 3 (71.9%)
IMAP assessments - 1,3-Propanediol, 2-amino-2-(hydroxymethyl)-: Environment tier I assessment
IMAP assessments - 1,3-Propanediol, 2-amino-2-(hydroxymethyl)-: Human health tier I assessment
M Chen, V Vijay, Q Shi, Z Liu, H Fang, W Tong. FDA-Approved Drug Labeling for the Study of Drug-Induced Liver Injury, Drug Discovery Today, 16(15-16):697-703, 2011. PMID:21624500 DOI:10.1016/j.drudis.2011.05.007
M Chen, A Suzuki, S Thakkar, K Yu, C Hu, W Tong. DILIrank: the largest reference drug list ranked by the risk for developing drug-induced liver injury in humans. Drug Discov Today 2016, 21(4): 648-653. PMID:26948801 DOI:10.1016/j.drudis.2016.02.015
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=LENZDBCJOHFCAS-UHFFFAOYSA-N
- Australian Industrial Chemicals Introduction Scheme (AICIS)1,3-Propanediol, 2-amino-2-(hydroxymethyl)-https://services.industrialchemicals.gov.au/search-assessments/1,3-Propanediol, 2-amino-2-(hydroxymethyl)-https://services.industrialchemicals.gov.au/search-inventory/
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/Tris(hydroxymethyl)aminomethanehttps://commonchemistry.cas.org/detail?cas_rn=77-86-1
- ChemIDplusTromethamine [USAN:USP]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000077861ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_useTromethaminehttps://www.drugbank.ca/drugs/DB03754
- DTP/NCILICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuse
- EPA Chemical Data Reporting (CDR)LICENSEThe U.S. Government retains a nonexclusive, royalty-free license to publish or reproduce these documents, or allow others to do so, for U.S. Government purposes. These documents may be freely distributed and used for non-commercial, scientific and educational purposes.https://www.epa.gov/web-policies-and-procedures/epa-disclaimers#copyright1,3-Propanediol, 2-amino-2-(hydroxymethyl)-https://www.epa.gov/chemical-data-reporting
- EPA Chemicals under the TSCA1,3-Propanediol, 2-amino-2-(hydroxymethyl)-https://www.epa.gov/chemicals-under-tscaEPA TSCA Classificationhttps://www.epa.gov/tsca-inventory
- EPA DSSToxTromethaminehttps://comptox.epa.gov/dashboard/DTXSID2023723CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeTrometamolhttps://chem.echa.europa.eu/100.000.969Trometamol (EC: 201-064-4)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/113562
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Hazardous Substances Data Bank (HSDB)
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingTromethaminehttp://www.hmdb.ca/metabolites/HMDB0240288HMDB0240288_msms_445751https://hmdb.ca/metabolites/HMDB0240288#spectra
- International Fragrance Association (IFRA)LICENSE(c) The International Fragrance Association, 2007-2021. All rights reserved.https://ifrafragrance.org/links/copyright2-Amino-2-(hydroxymethyl)-1,3-propanediolhttps://ifrafragrance.org/priorities/ingredients/ifra-transparency-list
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/2-amino-2-(hydroxymethyl)propane-1,3-diolhttps://www.epa.govt.nz/industry-areas/hazardous-substances/guidance-for-importers-and-manufacturers/hazardous-substances-databases/
- ChEBI
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licence
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- IUPAC Digitized pKa Datasetpropane, 2-amino-1,3-dihydroxy-2-hydroxymethyl-https://github.com/IUPAC/Dissociation-Constants
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jsp
- Drug Gene Interaction database (DGIdb)LICENSEThe data used in DGIdb is all open access and where possible made available as raw data dumps in the downloads section.http://www.dgidb.org/downloadsTROMETHAMINEhttps://www.dgidb.org/drugs/rxcui:10865
- Therapeutic Target Database (TTD)Tromethaminehttps://idrblab.net/ttd/data/drug/details/D03ZFN
- Consumer Product Information Database (CPID)LICENSECopyright (c) 2024 DeLima Associates. All rights reserved. Unless otherwise indicated, all materials from CPID are copyrighted by DeLima Associates. No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://www.whatsinproducts.com/contents/view/1/6Consumer Products Category Classificationhttps://www.whatsinproducts.com/
- Cosmetic Ingredient Review (CIR)
- EPA Chemical and Products Database (CPDat)EPA CPDat Classificationhttps://www.epa.gov/chemical-research/chemical-and-products-database-cpdat
- Haz-Map, Information on Hazardous Chemicals and Occupational DiseasesLICENSECopyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://haz-map.com/AboutTromethaminehttps://haz-map.com/Agents/18432
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/TROMETAMOLNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- Crystallography Open Database (COD)LICENSEAll data in the COD and the database itself are dedicated to the public domain and licensed under the CC0 License. Users of the data should acknowledge the original authors of the structural data.https://creativecommons.org/publicdomain/zero/1.0/
- The Cambridge Structural Database
- DailyMed
- Drug Induced Liver Injury Rank (DILIrank) DatasetLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Drugs@FDALICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingTROMETHAMINEhttps://www.accessdata.fda.gov/scripts/cder/daf/
- EU Clinical Trials Register
- FDA Orange BookLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- NMRShiftDB
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawTris(hydroxymethyl)aminomethanehttp://www.nist.gov/srd/nist1a.cfm
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlAnatomical Therapeutic Chemical (ATC) classificationhttp://www.genome.jp/kegg-bin/get_htext?br08303.keg
- Kruve Lab, Ionization & Mass Spectrometry, Stockholm Universitytrizma base
- MassBank Europe2-Amino-2-(hydroxymethyl)-1,3-propanediolhttps://massbank.eu/MassBank/Result.jsp?inchikey=LENZDBCJOHFCAS-UHFFFAOYSA-N
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- Metabolomics Workbench2-amino-2-methylol-propane-1,3-diolhttps://www.metabolomicsworkbench.org/data/StructureData.php?RegNo=71483
- National Drug Code (NDC) DirectoryLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Nature Chemical Biology
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- SpectraBase2-AMINO-2-HYDROXYMETHYLPROPANE-1,3-DIOLhttps://spectrabase.com/spectrum/HE6RYROdPmk1,3-PROPANEDIOL, 2-AMINO-2-(HYDROXYMETHYL)-https://spectrabase.com/spectrum/JqauZfQCpEhTRIS(HYDROXYMETHYL)AMINOMETHANE, 99%https://spectrabase.com/spectrum/6NemRBIDHcf2-AMINO-2-(HYDROXYMETHYL)-1,3-PROPANEDIOLhttps://spectrabase.com/spectrum/DAVlacXwzxe2-amino-2-(hydroxymethyl)-1,3-propanediolhttps://spectrabase.com/spectrum/4ZaLbsVGQg22-AMINO-2-(HYDROXYMETHYL)-1,3-PROPANEDIOLhttps://spectrabase.com/spectrum/CsEm9nId2Ns2-Amino-2-hydroxymethyl-1,3-propanediolhttps://spectrabase.com/spectrum/K9FkBgRhNdcTRIS(HYDROXYMETHYL)AMINOMETHANE, 99%https://spectrabase.com/spectrum/EqEJiTfJUjpTris(hydroxymethyl)aminomethanehttps://spectrabase.com/spectrum/AZQu7hId8FZ2-Amino-2-hydroxymethyl-1,3-propanediolhttps://spectrabase.com/spectrum/FNDOF9WaGJsTris(hydroxymethyl)aminomethanehttps://spectrabase.com/spectrum/3eX24Zaimg2-Amino-2-(hydroxymethyl)-1,3-propanediolhttps://spectrabase.com/spectrum/6Y1Owx0hzDqTris(hydroxymethyl)aminomethanehttps://spectrabase.com/spectrum/Netbx9tDeS
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.htmltromethaminehttps://rxnav.nlm.nih.gov/id/rxnorm/10865
- Springer Nature
- SpringerMaterialstris(hydroxymethyl)methanaminehttps://materials.springer.com/substanceprofile/docs/smsid_fcspbtrtvtgecwju
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- WHO Anatomical Therapeutic Chemical (ATC) ClassificationLICENSEUse of all or parts of the material requires reference to the WHO Collaborating Centre for Drug Statistics Methodology. Copying and distribution for commercial purposes is not allowed. Changing or manipulating the material is not allowed.https://www.whocc.no/copyright_disclaimer/
- Wikidatatromethaminehttps://www.wikidata.org/wiki/Q413961
- Wikipedia
- Wiley
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlTromethaminehttps://www.ncbi.nlm.nih.gov/mesh/68014325
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- International Agency for Research on Cancer (IARC)LICENSEMaterials made available by IARC/WHO enjoy copyright protection under the Berne Convention for the Protection of Literature and Artistic Works, under other international conventions, and under national laws on copyright and neighbouring rights. IARC exercises copyright over its Materials to make sure that they are used in accordance with the Agency's principles. All rights are reserved.https://publications.iarc.fr/Terms-Of-UseIARC Classificationhttps://www.iarc.fr/
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403030683https://pubchem.ncbi.nlm.nih.gov/substance/403030683
- NCBI