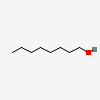
1-Octanol
- C8H18O
- CH3(CH2)6CH2OH
- 1-octanol
- Octan-1-ol
- octanol
- 111-87-5
- N-octanol
- Create:2004-09-16
- Modify:2025-01-18
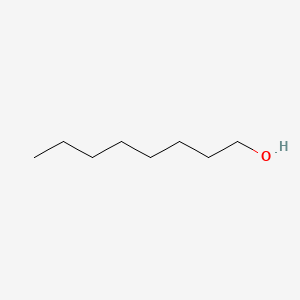
C8H18O
CH3(CH2)6CH2OH
- 1 Octanol
- 1-Octanol
- Alcohol, n-Octyl
- n Octanol
- n Octyl Alcohol
- n-Octanol
- n-Octyl Alcohol
- 1-octanol
- Octan-1-ol
- octanol
- 111-87-5
- N-octanol
- Octyl alcohol
- Capryl alcohol
- caprylic alcohol
- n-Octyl alcohol
- Heptyl carbinol
- 1-Hydroxyoctane
- Alcohol C-8
- Primary octyl alcohol
- n-Octan-1-ol
- Octilin
- n-Caprylic alcohol
- Alfol 8
- Sipol L8
- Lorol 20
- 1-Octyl alcohol
- Dytol M-83
- C8 alcohol
- n-Heptyl carbinol
- Octyl alcohol, normal-primary
- EPAL 8
- octyl-alcohol
- Octyl alcohol, primary
- N-octyl-alcohol
- Caswell No. 611A
- n-Capryl alcohol
- Octyl alcohol (natural)
- FEMA Number 2800
- Alcohol C8
- Lorol C 8-98
- NSC 9823
- FEMA No. 2800
- CCRIS 9099
- HSDB 700
- caprylyl alcohol
- 2-Capryl alcohol
- 1-n-octanol
- 1-Oktanol
- EINECS 203-917-6
- MFCD00002988
- Lorol C8
- EPA Pesticide Chemical Code 079037
- Octanol-(1)
- Prim-n-octyl alcohol
- DTXSID7021940
- UNII-NV1779205D
- CHEBI:16188
- AI3-02169
- ALFOL 8 ALCOHOL
- 2-Octanol ~99%
- Emery 3322
- Emery 3324
- NSC-9823
- 1-OCTANOL [FHFI]
- 1-OCTANOL [HSDB]
- 1-OCTANOL [MI]
- Octan-2-ol 98+ %
- Octyl alcohol normal-primary
- OCTYL ALCOHOL [FCC]
- KALCOHL-0898
- NACOL 8-99 ALCOHOL
- DTXCID101940
- N-OCTYL ALCOHOL, PRIMARY
- EC 203-917-6
- 1-OCTANOL-1,1-D2
- CO-898
- NV1779205D
- 220713-26-8
- CAS-111-87-5
- Octyl alcohol (n)
- Octanol (all isomers)
- octylalcohol
- Oktylalkohol
- nOctanol
- Oktanol
- 1Hydroxyoctane
- nOctyl alcohol
- nOctan1ol
- nCaprylic alcohol
- HEPTYLCARBINOL
- Dytol M83
- 1-Octanol (Standard)
- bmse000970
- bmse000980
- SCHEMBL8822
- Octyl alcohol, normalprimary
- WLN: Q8
- MLS001055318
- CHEMBL26215
- 1-Octanol, analytical standard
- GTPL4278
- CAPRYLYL ALCOHOL [INCI]
- BDBM22606
- CHEBI:37868
- 1-Octanol, anhydrous, >=99%
- NSC9823
- HMS3039O07
- 1-Octanol, for HPLC, >=99%
- HY-W032013R
- 1-Octanol, ACS reagent, >=99%
- 1-Octanol, ReagentPlus(R), 99%
- Tox21_201373
- Tox21_300096
- c0045
- LMFA05000130
- STL264193
- 1-Octanol, >=98%, FCC, FG
- 1-Octanol, natural, >=98%, FCC
- AKOS000120100
- DB12452
- HY-W032013
- USEPA/OPP Pesticide Code: 079037
- NCGC00091003-01
- NCGC00091003-02
- NCGC00091003-03
- NCGC00091003-04
- NCGC00091003-05
- NCGC00254099-01
- NCGC00258924-01
- BP-21329
- DA-59946
- LS-13539
- SMR000673567
- 1-Octanol, puriss., >=99.5% (GC)
- 1-Octanol, SAJ first grade, >=75.0%
- 1-Octanol, JIS special grade, >=98.0%
- 1-Octanol, Vetec(TM) reagent grade, 98%
- CS-0076037
- NS00010163
- O0036
- O0212
- EN300-19311
- C00756
- 1-Octanol, ACS spectrophotometric grade, >=99%
- Q161666
- J-002650
- BRD-K09338665-001-07-1
- F0001-0248
- Z104473500
- 958E4752-AAC3-4F72-A0BF-02D95F9E8071
- Boiling point
- Chemical diffusion
- Composition
- Crystal structure
- Density
- Diamagnetic susceptibility
- Dielectric constant
- Diffusion
- Diffusive flux
- Excess enthalpy
- Formula unit
- Fusion temperature
- Heat of solution
- Heat of sublimation
- Magnetic susceptibility
- Melting temperature
- Mixing enthalpy
- Optical coefficient
- Phase diagram
- Phase equilibrium
- Phase transition
- Refractive index
- Self-diffusion
- Sound absorption
- Sound propagation
- Sound velocity
- Space group
- Surface tension
- Thermal expansion coefficient
- Transition enthalpy
- Unit cell
- Unit cell parameter
- Vapor pressure
- Vapor-liquid equilibrium
- Virial coefficient
- Viscosity
56.0 99.99
41.0 88.90
55.0 81.80
43.0 71.60
70.0 59.70
56.0 99.99
55.0 86.89
41.0 81.58
43.0 70.15
69.0 62.51
56.0 1
41.0 0.89
55.0 0.82
43.0 0.72
70.0 0.60
56.0 1
55.0 0.87
41.0 0.82
43.0 0.70
69.0 0.63
72.07928 100
60.05512 36.07
114.10159 17.90
97.07317 4.93
55.02633 2.75
56 999
41 889
55 818
43 716
70 597
- Adipose Tissue
- Brain
- Epidermis
- Fibroblasts
- Intestine
- Placenta
- Skeletal Muscle
- Extracellular
- Membrane
- Lubricating agent
- Wetting agent (non-aqueous)
- Processing aids, not otherwise listed
- Paint additives and coating additives not described by other categories
- Adhesives and sealant chemicals
- Surfactant (surface active agent)
- Oxidizing/reducing agents
- Viscosity adjustors
- Not Known or Reasonably Ascertainable
- Intermediate
- Plasticizer
- Odor agents
- Other (specify)
- Surface active agents
- Adhesives and sealant chemicals
- Oxidizing/reducing agents
- Viscosity adjustors
- Lubricating agent
- Wetting agent (non-aqueous)
- Processing aids, not otherwise listed
- Other (specify)
- Fragrance
- Surface active agents
- Not Known or Reasonably Ascertainable
- Monomers
Information on 3 consumer products that contain Caprylic Alcohol in the following categories is provided:
• Auto Products
• Inside the Home
2019: 20,000,000 lb - <100,000,000 lb
2018: 20,000,000 lb - <100,000,000 lb
2017: 20,000,000 lb - <100,000,000 lb
2016: 20,000,000 lb - <100,000,000 lb
- Wholesale and Retail Trade
- Not Known or Reasonably Ascertainable
- Plastics Material and Resin Manufacturing
- All Other Basic Organic Chemical Manufacturing
- Oil and Gas Drilling, Extraction, and Support activities
- Agriculture, Forestry, Fishing and Hunting
- Paint and Coating Manufacturing
- All Other Chemical Product and Preparation Manufacturing
- Plastics Product Manufacturing

P264+P265, P280, P305+P351+P338, and P337+P317
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 9 reports by companies from 1 notifications to the ECHA C&L Inventory.
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Acute Tox. 4 (15.8%)
Acute Tox. 4 (15.8%)
Skin Irrit. 2 (23.8%)
Eye Irrit. 2A (98.7%)
Aquatic Chronic 3 (14.7%)

ERPG-1: 5 ppm - one hour exposure limit: 1 = mild transient health effects or objectionable odor [AIHA]
ERPG-2: 20 ppm - one hour exposure limit: 2 = impaired ability to take protective action [AIHA]
ERPG-3: 150 ppm - one hour exposure limit: 3 = life threatening health effects [AIHA]
Status: Active Update: 22-02-2023 https://echa.europa.eu/registration-dossier/-/registered-dossier/15210
Status: Active Update: 03-01-2019 https://echa.europa.eu/registration-dossier/-/registered-dossier/27226
IMAP assessments - Primary aliphatic alcohols (C7, C8): Human health tier II assessment
IMAP assessments - 1-Octanol: Environment tier I assessment
Neurotoxin - Acute solvent syndrome
Occupational hepatotoxin - Secondary hepatotoxins: the potential for toxic effect in the occupational setting is based on cases of poisoning by human ingestion or animal experimentation.
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=KBPLFHHGFOOTCA-UHFFFAOYSA-N
- Australian Industrial Chemicals Introduction Scheme (AICIS)
- CAMEO ChemicalsLICENSECAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data.https://cameochemicals.noaa.gov/help/reference/terms_and_conditions.htm?d_f=falseCAMEO Chemical Reactivity Classificationhttps://cameochemicals.noaa.gov/browse/react
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_useCaprylic alcoholhttps://www.drugbank.ca/drugs/DB12452
- DTP/NCILICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuse
- EPA Chemical Data Reporting (CDR)LICENSEThe U.S. Government retains a nonexclusive, royalty-free license to publish or reproduce these documents, or allow others to do so, for U.S. Government purposes. These documents may be freely distributed and used for non-commercial, scientific and educational purposes.https://www.epa.gov/web-policies-and-procedures/epa-disclaimers#copyright
- EPA Chemicals under the TSCAEPA TSCA Classificationhttps://www.epa.gov/tsca-inventory
- EPA DSSToxCompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeOctan-1-olhttps://chem.echa.europa.eu/100.003.561Octanol (EC: 249-405-6)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/104745Octan-1-ol (EC: 203-917-6)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/118013
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingCAPRYLIC ALCOHOLhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/NV1779205D
- Hazardous Substances Data Bank (HSDB)
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingHMDB0001183_cms_26831https://hmdb.ca/metabolites/HMDB0001183#spectra
- ILO-WHO International Chemical Safety Cards (ICSCs)
- International Fragrance Association (IFRA)LICENSE(c) The International Fragrance Association, 2007-2021. All rights reserved.https://ifrafragrance.org/links/copyright
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/
- Risk Assessment Information System (RAIS)LICENSEThis work has been sponsored by the U.S. Department of Energy (DOE), Office of Environmental Management, Oak Ridge Operations (ORO) Office through a joint collaboration between United Cleanup Oak Ridge LLC (UCOR), Oak Ridge National Laboratory (ORNL), and The University of Tennessee, Ecology and Evolutionary Biology, The Institute for Environmental Modeling (TIEM). All rights reserved.https://rais.ornl.gov/
- Haz-Map, Information on Hazardous Chemicals and Occupational DiseasesLICENSECopyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://haz-map.com/About1-Octanolhttps://haz-map.com/Agents/2916
- Joint FAO/WHO Expert Committee on Food Additives (JECFA)LICENSEPermission from WHO is not required for the use of WHO materials issued under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Intergovernmental Organization (CC BY-NC-SA 3.0 IGO) licence.https://www.who.int/about/policies/publishing/copyright
- ChEBI
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/LOTUS Treehttps://lotus.naturalproducts.net/
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licence
- Yeast Metabolome Database (YMDB)octan-1-olhttps://www.ymdb.ca/compounds/YMDB00808
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.html
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jsp
- IUPHAR/BPS Guide to PHARMACOLOGYLICENSEThe Guide to PHARMACOLOGY database is licensed under the Open Data Commons Open Database License (ODbL) https://opendatacommons.org/licenses/odbl/. Its contents are licensed under a Creative Commons Attribution-ShareAlike 4.0 International License (http://creativecommons.org/licenses/by-sa/4.0/)https://www.guidetopharmacology.org/about.jsp#licenseGuide to Pharmacology Target Classificationhttps://www.guidetopharmacology.org/targets.jsp
- Therapeutic Target Database (TTD)
- Consumer Product Information Database (CPID)LICENSECopyright (c) 2024 DeLima Associates. All rights reserved. Unless otherwise indicated, all materials from CPID are copyrighted by DeLima Associates. No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://www.whatsinproducts.com/contents/view/1/6Consumer Products Category Classificationhttps://www.whatsinproducts.com/
- Crystallography Open Database (COD)LICENSEAll data in the COD and the database itself are dedicated to the public domain and licensed under the CC0 License. Users of the data should acknowledge the original authors of the structural data.https://creativecommons.org/publicdomain/zero/1.0/
- The Cambridge Structural Database
- EPA Chemical and Products Database (CPDat)EPA CPDat Classificationhttps://www.epa.gov/chemical-research/chemical-and-products-database-cpdat
- EU Food Improvement Agents
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/1-OctanolNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- Hazardous Chemical Information System (HCIS), Safe Work Australia
- NITE-CMC1-Octanol - FY2012 (Revised classification)https://www.chem-info.nite.go.jp/chem/english/ghs/12-mhlw-2006e.html1-Octanol - FY2006 (New/original classication)https://www.chem-info.nite.go.jp/chem/english/ghs/06-imcg-0360e.html
- FDA Substances Added to FoodLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Flavor and Extract Manufacturers Association (FEMA)
- FooDBLICENSEFooDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (FooDB) and the original publication.https://foodb.ca/about1-Octanolhttps://foodb.ca/compounds/FDB012583
- NMRShiftDB
- MassBank Europe
- SpectraBase1-OCTAN-1,1-D2-OLhttps://spectrabase.com/spectrum/IZq75YwmN9v
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-law1-Octanolhttp://www.nist.gov/srd/nist1a.cfm
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.html
- KNApSAcK Species-Metabolite Database
- Natural Product Activity and Species Source (NPASS)
- The Natural Products AtlasLICENSEThe Natural Products Atlas is licensed under a Creative Commons Attribution 4.0 International License.https://www.npatlas.org/termsThe Natural Products Atlas Classificationhttps://www.npatlas.org/
- LIPID MAPSLipid Classificationhttps://www.lipidmaps.org/
- Metabolomics Workbench
- Nature Chemical Biology
- Nature Chemistry
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.html
- Protein Data Bank in Europe (PDBe)
- RCSB Protein Data Bank (RCSB PDB)LICENSEData files contained in the PDB archive (ftp://ftp.wwpdb.org) are free of all copyright restrictions and made fully and freely available for both non-commercial and commercial use. Users of the data should attribute the original authors of that structural data.https://www.rcsb.org/pages/policies
- Rhea - Annotated Reactions DatabaseLICENSERhea has chosen to apply the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/). This means that you are free to copy, distribute, display and make commercial use of the database in all legislations, provided you credit (cite) Rhea.https://www.rhea-db.org/help/license-disclaimer
- Springer Nature
- SpringerMaterials
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- USGS Health-Based Screening Levels for Evaluating Water-Quality DataLICENSEhttps://www.usgs.gov/legal
- Wikidata
- WikipediaManganese(II) titanatehttps://en.wikipedia.org/wiki/Manganese(II)_titanate
- Wiley
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.html
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403030130https://pubchem.ncbi.nlm.nih.gov/substance/403030130
- NCBI