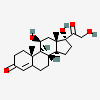
Hydrocortisone
- hydrocortisone
- Cortisol
- 50-23-7
- Acticort
- Cetacort
- Create:2004-09-16
- Modify:2025-01-18
 Hydrocortisone Acetate (annotation moved to); Hydrocortisone; polymyxin B sulfate (annotation moved to) ... View More ...
Hydrocortisone Acetate (annotation moved to); Hydrocortisone; polymyxin B sulfate (annotation moved to) ... View More ...
- 11 Epicortisol
- 11-Epicortisol
- Cortef
- Cortifair
- Cortisol
- Cortril
- Epicortisol
- Hydrocortisone
- Hydrocortisone, (11 alpha)-Isomer
- Hydrocortisone, (9 beta,10 alpha,11 alpha)-Isomer
- Pregn-4-ene-3,20-dione, 11,17,21-trihydroxy-, (11beta)-
- hydrocortisone
- Cortisol
- 50-23-7
- Acticort
- Cetacort
- Cortef
- Hydrasson
- Hydrocortisyl
- Hydrocortone
- Cobadex
- Hytone
- Cortenema
- Cortril
- Dermacort
- Proctocort
- Hycort
- Signef
- 17-Hydroxycorticosterone
- Optef
- Kendall's compound F
- Cort-Dome
- Cortanal
- Corticreme
- Cortifan
- Cortiment
- Cortispray
- Cortonema
- Dermolate
- Efcorbin
- Efcortelan
- Eldecort
- Ficortril
- Genacort
- Hycortol
- Hycortole
- Penecort
- Permicort
- Tarcortin
- Traumaide
- Alacort
- Cleiton
- Epicort
- Dihydrocostisone
- Hytone lotion
- Hidro-Colisona
- Hydro-Adreson
- Scheroson F
- Incortin-H
- Reichstein's substance M
- Ala-Scalp
- Domolene-HC
- Epiderm H
- Esiderm H
- Otosone-F
- Polcort H
- Aeroseb-HC
- Cortolotion
- Cortoxide
- Cremesone
- Eldercort
- Heb-Cort
- Maintasone
- Nutracort
- Delacort
- Dioderm
- Mildison
- Rectoid
- Synacort
- Anflam
- Hydrocorticosterone
- Hydroxycortisone
- H-Cort
- Hydro-Colisona
- Cortisol alcohol
- Incortin-hydrogen
- Ala-Cort
- Barseb HC
- Dermocortal
- Flexicort
- Texacort
- Timocort
- Evacort
- Komed HC
- Hydrocortisone base
- Lacticare-HC
- Texacort lotion 25
- Hydrocortisone alcohol
- Hidrocortisona
- Algicirtis
- Aquacort
- Colocort
- Cortesal
- Cortisolonum
- Hidalone
- Hytisone
- Kyypakkaus
- Lactisona
- Lubricort
- Meusicort
- Milliderm
- Sanatison
- Schericur
- Sigmacort
- Stiefcorcil
- Amberin
- Cutisol
- Dermil
- Glycort
- Uniderm
- Foille Insetti
- Gyno-Cortisone
- Balneol-hc
- Transderma H
- Basan-Corti
- Clear aid
- Cremicort-H
- Dome-cort
- Stie-cort
- Beta-hc
- Neosporin-H Ear
- Remederm HC
- Aquanil HC
- Cortisporin Otico
- Derm-Aid
- Heb Cort
- Nogenic HC
- Scalpicin Capilar
- Systral Hydrocort
- Prevex HC
- Cortisporin
- Efcortelin
- Fiocortril
- Hydrocortisone free alcohol
- Hydrocortisonum
- Proctofoam
- Hydracort
- Medicort
- Otocort
- Zenoxone
- Drotic
- Vytone
- 11beta-Hydroxycortisone
- Nystaform-HC
- Aeroseb HC
- CaldeCORT Spray
- Anusol HC
- Pediotic Suspension
- Idrocortisone
- 17alpha-Hydroxycorticosterone
- Alphaderm
- Hydrocortal
- Hydroskin
- Otalgine
- Otobiotic
- Plenadren
- Protocort
- Hysone
- Hydrocortisone (Cortisol)
- Ef corlin
- 11beta-Hydrocortisone
- Compound F
- Lacticare HC
- Compound F (kendall)
- 11-beta-Hydrocortisone
- 11-beta-Hydroxycortisone
- VoSol HC
- Chronocort
- Preparation H Hydrocortisone Cream
- Neo-Cort-Dome
- 11beta,17alpha,21-Trihydroxy-4-pregnene-3,20-dione
- 11beta,17alpha,21-Trihydroxypregn-4-ene-3,20-dione
- Otic-Neo-Cort-Dome
- 11beta,17,21-Trihydroxypregn-4-ene-3,20-dione
- NSC 10483
- HC
- [3H]cortisol
- NSC-10483
- Prestwick_265
- 4-Pregnene-11beta,17alpha,21-triol-3,20-dione
- CHEBI:17650
- (11beta)-11,17,21-trihydroxypregn-4-ene-3,20-dione
- NSC10483
- 11.beta.-Hydrocortisone
- Dermaspray
- Hydrocortisone (Standard)
- MFCD00011654
- WI4X0X7BPJ
- (8S,9S,10R,11S,13S,14S,17R)-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-3-one
- 11beta,17,21-Trihydroxyprogesterone
- 11.beta.-Hydroxycortisone
- Ophthocort
- Terra-cortril
- MLS000069609
- 17.alpha.-Hydroxycorticosterone
- DTXSID7020714
- Pregn-4-ene-3,20-dione, 11,17,21-trihydroxy-, (11.beta.)-
- Pregn-4-ene-3,20-dione, 11,17,21-trihydroxy-, (11beta)-
- 4-Pregnen-11beta,17alpha,21-triol-3,20-dione
- Idrocortisone [DCIT]
- Genacort (lotion)
- Anucort
- hydrocortisone(cortisol)
- Prepcort
- SMR000059022
- Hydrocortisonum [INN-Latin]
- Proctozone HC
- Scalp-Cort
- Hidrocortisona [INN-Spanish]
- Rectasol-HC
- Hydro-RX
- (8S,9S,10R,11S,13S,14S,17R)-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3(2H)-one
- DTXCID10714
- HC (HYDROCORTISONE)
- 11.beta.,17,21-Trihydroxypregn-4-ene-3,20-dione
- Corhydron
- (11alpha,14beta)-11,17,21-Trihydroxypregn-4-Ene-3,20-Dione
- DuoCort
- HYDROCORTISONE IN ABSORBASE
- Proctosol-HC
- HC #1
- HC #4
- Acticort (TN)
- Colocort (TN)
- SMR000653523
- Cortef (TN)
- Hytone (TN)
- CCRIS 5854
- component of Otalgine
- Anusol HC (TN)
- component of Lubricort
- COR-OTICIN
- HSDB 3339
- EINECS 200-020-1
- UNII-WI4X0X7BPJ
- component of Neo-Cort-Dome
- Cortizol
- Efmody
- AI3-25006
- 3h-cortisol
- 11beta-cortisol
- CAS-50-23-7
- 11-Hydrocortisone
- Plenadren (TN)
- NCGC00022848-06
- 11b-Hydrocortisone
- Kendalls compound F
- Hydrocortisone [USP:INN:BAN:JAN]
- Drotic (Salt/Mix)
- 11b-Hydroxycortisone
- Otocort (Salt/Mix)
- Pregn-4-ene-3,20-dione, 11beta,17,21-trihydroxy-
- Otalgine (Salt/Mix)
- Hydrocortisone, 98%
- 11,17,21-Trihydroxypregn-4-ene-3,20-dione
- ALKINDI SPRINKLE
- Alphaderm (Salt/Mix)
- Hydrocortisone, topical
- Otobiotic (Salt/Mix)
- Reichsteins substance M
- 4p6x
- Pregn-4-ene-3,20-dione, 11,17,21-trihydroxy-, (11b)-
- Cort-Quin (Salt/Mix)
- Cortisporin (Salt/Mix)
- VoSol HC (Salt/Mix)
- 11a-Hydroxycorticosterone
- 17a-Hydroxycorticosterone
- Opera_ID_1292
- Pregn-4-ene-3,20-dione, 11,17,21-trihydroxy-, (11-beta)-
- Prestwick0_000447
- Prestwick1_000447
- Prestwick2_000447
- Prestwick3_000447
- Epitope ID:174851
- UPCMLD-DP133
- EC 200-020-1
- H 4001
- HYDROCORTISONE [II]
- HYDROCORTISONE [MI]
- SCHEMBL4148
- Neo-Cort-Dome (Salt/Mix)
- HYDROCORTISONE [INN]
- HYDROCORTISONE [JAN]
- Lopac0_000594
- 11alpha-Hydroxycorticosterone
- BSPBio_000494
- HYDROCORTISONE [HSDB]
- MLS001148103
- MLS002207135
- MLS002222189
- MLS002548868
- HYDROCORTISONE [VANDF]
- Cortisol, 1mg/ml in Methanol
- SPBio_002433
- HYDROCORTISONE [MART.]
- BPBio1_000544
- CHEMBL389621
- GTPL2868
- HYDROCORTISONE [USP-RS]
- HYDROCORTISONE [WHO-DD]
- HYDROCORTISONE [WHO-IP]
- Pediotic Suspension (Salt/Mix)
- UPCMLD-DP133:001
- BDBM13775
- HY-N0583R
- Otic-Neo-Cort-Dome (Salt/Mix)
- 2v95
- Hydrocortisone (JP17/USP/INN)
- HMS1569I16
- HMS2090M04
- HMS2096I16
- HMS2230B18
- HMS2235F17
- HMS3259C05
- HMS3261H10
- HMS3713I16
- Hydrocortisone, >=98% (HPLC)
- Vioform-Hydrocortisone (Salt/Mix)
- 11b,17,21-Trihydroxyprogesterone
- HYDROCORTISONE [GREEN BOOK]
- BCP09054
- HY-N0583
- HYDROCORTISONE [ORANGE BOOK]
- Tox21_110883
- Tox21_200815
- Tox21_500594
- HYDROCORTISONE [EP MONOGRAPH]
- LMST02030001
- s1696
- HYDROCORTISONE [USP MONOGRAPH]
- AKOS001582651
- HYDROCORTISONUM [WHO-IP LATIN]
- OTICAIR COMPONENT HYDROCORTISONE
- OTOCORT COMPONENT HYDROCORTISONE
- Tox21_110883_1
- 1ST2226
- CCG-204683
- DB00741
- LP00594
- NC00456
- SDCCGSBI-0050576.P003
- 11.beta.,17,21-trihydroxyprogesterone
- CIPRO HC COMPONENT HYDROCORTISONE
- ORLEX HC COMPONENT HYDROCORTISONE
- OTOBIONE COMPONENT HYDROCORTISONE
- PEDIOTIC COMPONENT HYDROCORTISONE
- PYOCIDIN COMPONENT HYDROCORTISONE
- SMP1_000156
- VOSOL HC COMPONENT HYDROCORTISONE
- ALPHADERM COMPONENT HYDROCORTISONE
- NCGC00022848-07
- NCGC00022848-09
- NCGC00022848-10
- NCGC00022848-11
- NCGC00022848-12
- NCGC00022848-13
- NCGC00022848-14
- NCGC00022848-15
- NCGC00022848-17
- NCGC00022848-26
- NCGC00258369-01
- NCGC00261279-01
- OTOBIOTIC COMPONENT HYDROCORTISONE
- (1S,10S,11S,15S,17S,2R,14R)-14,17-dihydroxy-14-(2-hydroxyacetyl)-2,15-dimethyl tetracyclo[8.7.0.0<2,7>.0<11,15>]heptadec-6-en-5-one
- AC-12902
- AS-11651
- BP-20390
- NCI60_000118
- ACETASOL HC COMPONENT HYDROCORTISONE
- CALMURID HC COMPONENT HYDROCORTISONE
- 4-Pregnene-11alpha,21-triol 3,20-dione
- CS-0694892
- EU-0100594
- NS00000570
- PREDNISOLONE IMPURITY A [EP IMPURITY]
- 4-Pregnene-11b,17a,21-triol-3,20-dione
- C00735
- D00088
- EN300-120630
- U 1851
- Hydrocortisone, meets USP testing specifications
- Pregn-4-ene-3, 11.beta.,17,21-trihydroxy-
- A929789
- Hydrocortisone, VETRANAL(TM), analytical standard
- Q190875
- SR-01000000139
- Q-201211
- SR-01000000139-3
- BRD-K93568044-001-03-1
- BRD-K93568044-001-11-4
- BRD-K93568044-001-32-0
- HYDROCORTISONE ACETATE IMPURITY A [EP IMPURITY]
- Hydrocortisone, BioReagent, suitable for cell culture
- 4-Pregnen-11.beta.,17.alpha.,21-triol-3,20-dione
- 4-Pregnene-11.beta.,17.alpha.,21-triol-3,20-dione
- Pregn-4-ene-3,20-dione, 11.beta.,17,21-trihydroxy-
- Z1530425064
- (11beta)-11,17,21-Trihydroxy-pregn-4-ene-3,20-dione
- 11.beta.,17.alpha.,21-Trihydroxy-4-pregnene-3,20-dione
- 11.beta.,17.alpha.,21-Trihydroxypregn-4-ene-3,20-dione
- B48448A1-24BA-47CA-8D9E-43E5BC949386
- Hydrocortisone, British Pharmacopoeia (BP) Assay Standard
- Pregn-4-ene-3, 11,17,21-trihydroxy-, (11.beta.)-
- 11,17,21-Trihydroxypregn-4-ene-3,20-dione, (11.beta.)-
- HYDROCORTISONE SODIUM SUCCINATE IMPURITY A [EP IMPURITY]
- Hydrocortisone, European Pharmacopoeia (EP) Reference Standard
- HYDROCORTISONE HYDROGEN SUCCINATE IMPURITY A [EP IMPURITY]
- Hydrocortisone, United States Pharmacopeia (USP) Reference Standard
- Hydrocortisone-Water Soluble, BioReagent, suitable for cell culture
- Hydrocortisone, gamma-irradiated, powder, BioXtra, suitable for cell culture
- Hydrocortisone for peak identification, European Pharmacopoeia (EP) Reference Standard
- Hydrocortisone, Pharmaceutical Secondary Standard; Certified Reference Material
- (10R,13S,17R)-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-3-one
- (1R,3aS,3bS,9aR,9bS,10S,11aS)-1,10-dihydroxy-1-(2-hydroxyacetyl)-9a,11a-dimethyl-1H,2H,3H,3aH,3bH,4H,5H,7H,8H,9H,9aH,9bH,10H,11H,11aH-cyclopenta[a]phenanthren-7-one
- (1S,2R,10S,11S,14R,15S,17S)-14,17-dihydroxy-14-(2-hydroxyacetyl)-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-5-one
212.9 Ų [M+Na]+ [CCS Type: DT; Method: single field calibrated with ESI Low Concentration Tuning Mix (Agilent)]
210.7 Ų [M+K]+ [CCS Type: DT; Method: single field calibrated with ESI Low Concentration Tuning Mix (Agilent)]
191.9 Ų [M-H]- [CCS Type: DT; Method: single field calibrated with ESI Low Concentration Tuning Mix (Agilent)]
188.6 Ų [M+H]+ [CCS Type: DT; Method: single field calibrated with ESI Low Concentration Tuning Mix (Agilent)]
189.27 Ų [M+H]+ [CCS Type: DT; Method: stepped-field]
213.72 Ų [M+Na]+ [CCS Type: DT; Method: stepped-field]
184.11 Ų [M+H]+ [CCS Type: TW; Method: calibrated with polyalanine and drug standards]
180.18 Ų [M+H-H2O]+ [CCS Type: TW; Method: calibrated with polyalanine and drug standards]
182.99 Ų [M-H2O-H]- [CCS Type: DT; Method: single field calibrated with Agilent tune mix (Agilent)]
189.1 Ų [M+H]+ [CCS Type: DT; Method: single field calibrated with Agilent tune mix (Agilent)]
213.91 Ų [M+Na]+ [CCS Type: DT; Method: stepped-field]
188.88 Ų [M+H]+ [CCS Type: DT; Method: stepped-field]
192.1 Ų [M-H]-
188.9 Ų [M+H]+
213.2 Ų [M+K]+
211.6 Ų [M+Na]+
188.95 Ų [M+Cl]-
191.54 Ų [M+HCOO]-
187.79 Ų [M+H]+
191.98 Ų [M-H]-
Adrenal hormones and synthetic substitutes
Anti-inflammatory medicines
Antiallergics and medicines used in anaphylaxis
Dermatological medicines > Anti-inflammatory and antipruritic medicines
Hormones and antihormones
Ophthalmological preparations > Anti-inflammatory agents
103.0 1
91.0 0.64
84.0 0.63
89.0 0.61
105.0 0.47
91.0 100
105.0 47.65
93.0 41.34
152.0 34.23
117.0 28.43
363.2158 100
327.1947 33.42
364.2197 25.12
309.184 20.66
121.0639 18.26
121.064 100
259.1689 57.16
145.1 46.95
131.0842 43.84
143.0841 39.24
121.065 999
97.0646 333
363.2144 292
267.1743 262
309.1838 260
363.2167 999
121.0646 312
309.185 164
327.1937 137
345.2049 128
332 999
362 677
302 632
163 608
344 571
- Acetic acid, glacial; hydrocortisone (annotation moved to)
Hydrocortisone Acetate (annotation moved to)
- Hydrocortisone; polymyxin B sulfate (annotation moved to)
- Hydrocortisone; neomycin sulfate; polymyxin B sulfate (annotation moved to)
- Chloramphenicol; hydrocortisone acetate; polymyxin B sulfate (annotation moved to)
- Hydrocortisone Acetate; Neomycin Sulfate (annotation moved to)
D - Dermatologicals
D07 - Corticosteroids, dermatological preparations
D07X - Corticosteroids, other combinations
D07XA - Corticosteroids, weak, other combinations
D07XA01 - Hydrocortisone
S - Sensory organs
S01 - Ophthalmologicals
S01B - Antiinflammatory agents
S01BA - Corticosteroids, plain
S01BA02 - Hydrocortisone
S - Sensory organs
S02 - Otologicals
S02B - Corticosteroids
S02BA - Corticosteroids
S02BA01 - Hydrocortisone
S - Sensory organs
S01 - Ophthalmologicals
S01C - Antiinflammatory agents and antiinfectives in combination
S01CB - Corticosteroids/antiinfectives/mydriatics in combination
S01CB03 - Hydrocortisone
A - Alimentary tract and metabolism
A01 - Stomatological preparations
A01A - Stomatological preparations
A01AC - Corticosteroids for local oral treatment
A01AC03 - Hydrocortisone
C - Cardiovascular system
C05 - Vasoprotectives
C05A - Agents for treatment of hemorrhoids and anal fissures for topical use
C05AA - Corticosteroids
C05AA01 - Hydrocortisone
D - Dermatologicals
D07 - Corticosteroids, dermatological preparations
D07A - Corticosteroids, plain
D07AA - Corticosteroids, weak (group i)
D07AA02 - Hydrocortisone
H - Systemic hormonal preparations, excl. sex hormones and insulins
H02 - Corticosteroids for systemic use
H02A - Corticosteroids for systemic use, plain
H02AB - Glucocorticoids
H02AB09 - Hydrocortisone
A - Alimentary tract and metabolism
A07 - Antidiarrheals, intestinal antiinflammatory/antiinfective agents
A07E - Intestinal antiinflammatory agents
A07EA - Corticosteroids acting locally
A07EA02 - Hydrocortisone
- Adipose Tissue
- Adrenal Cortex
- Adrenal Gland
- Adrenal Medulla
- Bladder
- Brain
- Epidermis
- Fibroblasts
- Intestine
- Kidney
- Leukocyte
- Liver
- Neuron
- Ovary
- Pancreas
- Placenta
- Platelet
- Prostate
- Skeletal Muscle
- Spleen
- Testis
- Cytoplasm
- Endoplasmic reticulum
- Extracellular
- Membrane
- Mitochondria
- 11-beta-hydroxylase deficiency (CYP11B1)
- 17-alpha-hydroxylase deficiency (CYP17)
- 21-hydroxylase deficiency (CYP21)
- 3-Beta-Hydroxysteroid Dehydrogenase Deficiency
- Adrenal Hyperplasia Type 3 or Congenital Adrenal Hyperplasia due to 21-hydroxylase Deficiency
- Adrenal Hyperplasia Type 5 or Congenital Adrenal Hyperplasia due to 17 Alpha-hydroxylase Deficiency
- Apparent mineralocorticoid excess syndrome
- Congenital Lipoid Adrenal Hyperplasia (CLAH) or Lipoid CAH
- Corticosterone methyl oxidase I deficiency (CMO I)
- Corticosterone methyl oxidase II deficiency - CMO II
- Total 12 pathways, visit the HMDB page for details
Use (kg; approx.) in Germany (2009): >1000
Use (kg) in USA (2002): 21000
Use (kg) in France (2004): 453
Consumption (g per capita; approx.) in Germany (2009): 0.0122
Consumption (g per capita) in the USA (2002): 0.0744
Consumption (g per capita) in France (2004): 0.0075
Excretion rate: 0.06
Calculated removal (%): 45.9
Information on 6 consumer products that contain Hydrocortisone in the following categories is provided:
• Personal Care
• Pet Care

H361 (86.8%): Suspected of damaging fertility or the unborn child [Warning Reproductive toxicity]
H373 (31.5%): May causes damage to organs through prolonged or repeated exposure [Warning Specific target organ toxicity, repeated exposure]
P203, P260, P280, P318, P319, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 273 reports by companies from 33 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Reported as not meeting GHS hazard criteria per 3 of 273 reports by companies. For more detailed information, please visit ECHA C&L website.
There are 32 notifications provided by 270 of 273 reports by companies with hazard statement code(s).
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Repr. 2 (86.8%)
STOT RE 2 (31.5%)
IMAP assessments - Pregn-4-ene-3,20-dione, 11,17,21-trihydroxy-, (11.beta.)-: Human health tier I assessment
IMAP assessments - Pregn-4-ene-3,20-dione, 11,17,21-trihydroxy-, (11.beta.)-: Environment tier I assessment
M Chen, V Vijay, Q Shi, Z Liu, H Fang, W Tong. FDA-Approved Drug Labeling for the Study of Drug-Induced Liver Injury, Drug Discovery Today, 16(15-16):697-703, 2011. PMID:21624500 DOI:10.1016/j.drudis.2011.05.007
M Chen, A Suzuki, S Thakkar, K Yu, C Hu, W Tong. DILIrank: the largest reference drug list ranked by the risk for developing drug-induced liver injury in humans. Drug Discov Today 2016, 21(4): 648-653. PMID:26948801 DOI:10.1016/j.drudis.2016.02.015
◉ Summary of Use during Lactation
Hydrocortisone (cortisol) is a normal component of breastmilk, but it has not been studied in milk after exogenous administration in pharmacologic amounts. Although it is unlikely that dangerous amounts of hydrocortisone would reach the infant, a better studied corticosteroid might be preferred. Maternal use of hydrocortisone as an enema would not be expected to cause any adverse effects in breastfed infants. Local maternal injections, such as for tendinitis, would not be expected to cause any adverse effects in breastfed infants. Medium to large doses of corticosteroids given systemically or injected into joints or the breast have been reported to cause temporary reduction of lactation. See also Hydrocortisone, Topical.
Cortisol in breastmilk might have a role in intestinal maturation, the intestinal microbiome, growth, body composition or neurodevelopment, but adequate studies are lacking. Concentrations follow a diurnal rhythm, with the highest concentrations in the morning at about 7:00 am and the lowest concentrations in the late afternoon and evening. Cortisol concentration in milk also increase with infant age and decrease with complementary feeding and infant illness. Cortisol in milk may protect against later infant obesity, especially in girls; however, in another study, milk glucocorticoid levels were positively associated with percent fat mass, adiposity and head circumference at 1 year of age. Maternal stress can increase breastmilk cortisol levels, especially with preterm births. Some information indicates that maternal adverse childhood experiences may decrease cortisol concentration in their breastmilk.
◉ Effects in Breastfed Infants
None reported with any systemic corticosteroid.
◉ Effects on Lactation and Breastmilk
Published information on the effects of hydrocortisone on serum prolactin or on lactation in nursing mothers was not found as of the revision date. Medium to large doses of corticosteroids given systemically or injected into joints or the breast have been reported to cause temporary reduction of lactation.
A study of 46 women who delivered an infant before 34 weeks of gestation found that a course of another corticosteroid (betamethasone, 2 intramuscular injections of 11.4 mg of betamethasone 24 hours apart) given between 3 and 9 days before delivery resulted in delayed lactogenesis II and lower average milk volumes during the 10 days after delivery. Milk volume was not affected if the infant was delivered less than 3 days or more than 10 days after the mother received the corticosteroid. An equivalent dosage regimen of hydrocortisone might have the same effect.
A study of 87 pregnant women found that betamethasone given as above during pregnancy caused a premature stimulation of lactose secretion during pregnancy. Although the increase was statistically significant, the clinical importance appears to be minimal. An equivalent dosage regimen of hydrocortisone might have the same effect.
◉ Summary of Use during Lactation
Topical hydrocortisone has not been studied during breastfeeding. Since only extensive application of the most potent corticosteroids may cause systemic effects in the mother, it is unlikely that short-term application of topical hydrocortisone would pose a risk to the breastfed infant by passage into breastmilk. However, it would be prudent to use the least potent drug on the smallest area of skin possible. It is important to ensure that the infant's skin does not come into direct contact with the areas of skin that have been treated. Current guidelines allow topical corticosteroids to be applied to the nipples just after nursing for eczema, with the nipples cleaned gently before nursing. Only water-miscible cream or gel products should be applied to the breast because ointments may expose the infant to high levels of mineral paraffins via licking. Maternal use rectally with a cream or by suppository poses little to no risk to the breastfed infant.
◉ Effects in Breastfed Infants
Topical application of a corticosteroid with relatively high mineralocorticoid activity (isofluprednone acetate) to the mother's nipples resulted in prolonged QT interval, cushingoid appearance, severe hypertension, decreased growth and electrolyte abnormalities in her 2-month-old breastfed infant. The mother had used the cream since birth for painful nipples.
◉ Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
PubMed: 25870516, 210721
MetaGene: Metabolic & Genetic Information Center (MIC: http://www.metagene.de)
PubMed: 6157502, 11129331, 28583327, 15505778, 25557019, 23705938, 1886403, 12072887, 19893767
MetaGene: Metabolic & Genetic Information Center (MIC: http://www.metagene.de)
PubMed: 6589104, 16277678, 15338487, 10361015, 15249323
Tie-juan ShaoZhi-xing HeZhi-jun XieHai-chang LiMei-jiao WangCheng-ping Wen. Characterization of ankylosing spondylitis and rheumatoid arthritis using 1H NMR-based metabolomics of human fecal extracts. Metabolomics. April 2016, 12:70: https://link.springer.com/article/10.1007/s11306-016-1000-2
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=JYGXADMDTFJGBT-VWUMJDOOSA-N
- Australian Industrial Chemicals Introduction Scheme (AICIS)Pregn-4-ene-3,20-dione, 11,17,21-trihydroxy-, (11.beta.)-https://services.industrialchemicals.gov.au/search-assessments/Pregn-4-ene-3,20-dione, 11,17,21-trihydroxy-, (11.beta.)-https://services.industrialchemicals.gov.au/search-inventory/
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusHydrocortisone [USP:INN:BAN:JAN]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000050237ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_useHydrocortisonehttps://www.drugbank.ca/drugs/DB00741
- DTP/NCILICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuse
- EPA Chemicals under the TSCAPregn-4-ene-3,20-dione, 11,17,21-trihydroxy-, (11.beta.)-https://www.epa.gov/chemicals-under-tscaEPA TSCA Classificationhttps://www.epa.gov/tsca-inventory
- EPA DSSToxHydrocortisonehttps://comptox.epa.gov/dashboard/DTXSID7020714CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeHydrocortisonehttps://chem.echa.europa.eu/100.000.019Hydrocortisone (EC: 200-020-1)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/48986
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Hazardous Substances Data Bank (HSDB)HYDROCORTISONEhttps://pubchem.ncbi.nlm.nih.gov/source/hsdb/3339
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingHMDB0000063_cms_31583https://hmdb.ca/metabolites/HMDB0000063#spectra
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/
- BindingDBLICENSEAll data curated by BindingDB staff are provided under the Creative Commons Attribution 3.0 License (https://creativecommons.org/licenses/by/3.0/us/).https://www.bindingdb.org/rwd/bind/info.jsp(1S,2R,10S,11S,14R,15S,17S)-14,17-dihydroxy-14-(2-hydroxyacetyl)-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-5-onehttps://www.bindingdb.org/rwd/bind/chemsearch/marvin/MolStructure.jsp?monomerid=13775
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jspHydrocortisonehttps://ctdbase.org/detail.go?type=chem&acc=D006854
- IUPHAR/BPS Guide to PHARMACOLOGYLICENSEThe Guide to PHARMACOLOGY database is licensed under the Open Data Commons Open Database License (ODbL) https://opendatacommons.org/licenses/odbl/. Its contents are licensed under a Creative Commons Attribution-ShareAlike 4.0 International License (http://creativecommons.org/licenses/by-sa/4.0/)https://www.guidetopharmacology.org/about.jsp#licenseGuide to Pharmacology Target Classificationhttps://www.guidetopharmacology.org/targets.jsp
- Therapeutic Target Database (TTD)Hydrocortisonehttps://idrblab.net/ttd/data/drug/details/D0KR5BHydrocortisonehttps://idrblab.net/ttd/data/drug/details/D0FA3O
- CCSbaseCCSbase Classificationhttps://ccsbase.net/
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/HydrocortisoneNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- ChEBI
- FDA Pharm ClassesLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingFDA Pharmacological Classificationhttps://www.fda.gov/ForIndustry/DataStandards/StructuredProductLabeling/ucm162549.htm
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/Hydrocortisonehttps://www.wikidata.org/wiki/Q190875LOTUS Treehttps://lotus.naturalproducts.net/
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licenceHYDROCORTISONEhttps://platform.opentargets.org/drug/CHEMBL389621
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- Consumer Product Information Database (CPID)LICENSECopyright (c) 2024 DeLima Associates. All rights reserved. Unless otherwise indicated, all materials from CPID are copyrighted by DeLima Associates. No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://www.whatsinproducts.com/contents/view/1/6Consumer Products Category Classificationhttps://www.whatsinproducts.com/
- Crystallography Open Database (COD)LICENSEAll data in the COD and the database itself are dedicated to the public domain and licensed under the CC0 License. Users of the data should acknowledge the original authors of the structural data.https://creativecommons.org/publicdomain/zero/1.0/
- DailyMed
- Drug Induced Liver Injury Rank (DILIrank) DatasetLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- European Medicines Agency (EMA)LICENSEInformation on the European Medicines Agency's (EMA) website is subject to a disclaimer and copyright and limited reproduction notices.https://www.ema.europa.eu/en/about-us/legal-noticePlenadren (EMEA/H/C/002185)https://www.ema.europa.eu/en/medicines/human/EPAR/plenadrenEfmody (EMEA/H/C/005105)https://www.ema.europa.eu/en/medicines/human/EPAR/efmodyAlkindi (EMEA/H/C/004416)https://www.ema.europa.eu/en/medicines/human/EPAR/alkindihydrocortisone (P/0031/2013)https://www.ema.europa.eu/en/medicines/human/paediatric-investigation-plans/emea-001283-pip01-12
- Drugs and Lactation Database (LactMed)Hydrocortisonehttps://www.ncbi.nlm.nih.gov/books/n/lactmed/LM138/Hydrocortisone, Topicalhttps://www.ncbi.nlm.nih.gov/books/n/lactmed/LM405/
- Drugs@FDALICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- WHO Model Lists of Essential MedicinesLICENSEPermission from WHO is not required for the use of WHO materials issued under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Intergovernmental Organization (CC BY-NC-SA 3.0 IGO) license.https://www.who.int/about/policies/publishing/copyrightHydrocortisonehttps://list.essentialmeds.org/medicines/33
- ECI Group, LCSB, University of LuxembourgCortisol
- Natural Product Activity and Species Source (NPASS)
- EPA Chemical and Products Database (CPDat)EPA CPDat Classificationhttps://www.epa.gov/chemical-research/chemical-and-products-database-cpdat
- EU Clinical Trials Register
- FDA Approved Animal Drug Products (Green Book)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- FDA Orange BookLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- WHO Anatomical Therapeutic Chemical (ATC) ClassificationLICENSEUse of all or parts of the material requires reference to the WHO Collaborating Centre for Drug Statistics Methodology. Copying and distribution for commercial purposes is not allowed. Changing or manipulating the material is not allowed.https://www.whocc.no/copyright_disclaimer/Hydrocortisonehttps://www.whocc.no/atc_ddd_index/?code=D07XA01Hydrocortisonehttps://www.whocc.no/atc_ddd_index/?code=S01BA02Hydrocortisonehttps://www.whocc.no/atc_ddd_index/?code=S02BA01Hydrocortisonehttps://www.whocc.no/atc_ddd_index/?code=S01CB03Hydrocortisonehttps://www.whocc.no/atc_ddd_index/?code=A01AC03Hydrocortisonehttps://www.whocc.no/atc_ddd_index/?code=C05AA01Hydrocortisonehttps://www.whocc.no/atc_ddd_index/?code=D07AA02Hydrocortisonehttps://www.whocc.no/atc_ddd_index/?code=H02AB09Hydrocortisonehttps://www.whocc.no/atc_ddd_index/?code=A07EA02
- FDA Medication GuidesLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingALKINDI SPRINKLEhttps://dps.fda.gov/medguide
- National Drug Code (NDC) DirectoryLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- MassBank Europe(8S,9S,10R,11S,13S,14S,17R)-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-3-onehttps://massbank.eu/MassBank/Result.jsp?inchikey=JYGXADMDTFJGBT-VWUMJDOOSA-N
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawHydrocortisonehttp://www.nist.gov/srd/nist1a.cfm
- SpectraBaseHydrocortisonehttps://spectrabase.com/spectrum/2r1oIq8trPnHydrocortisonehttps://spectrabase.com/spectrum/B3pRb7i0mq84-Pregnen-11β,17,21-triol-3, 20-dionehttps://spectrabase.com/spectrum/4R0FNbbkQuIHydrocortisonehttps://spectrabase.com/spectrum/2LPE9VhtEN411-BETA-17,21-TRIHYDROXYPREGN-4-EN-3,20-DION,(CORTISOL)https://spectrabase.com/spectrum/IQaopr5BkXP4-Pregnen-11β,17,21-triol-3,20-dionehttps://spectrabase.com/spectrum/FhOIEJ8nutuHydrocortisonehttps://spectrabase.com/spectrum/93RL93MMWzjHydrocortisonehttps://spectrabase.com/spectrum/6qBAIjBUk8S
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlCompounds with biological roleshttp://www.genome.jp/kegg-bin/get_htext?br08001.kegTherapeutic category of drugs in Japanhttp://www.genome.jp/kegg-bin/get_htext?br08301.kegUSP drug classificationhttp://www.genome.jp/kegg-bin/get_htext?br08302.kegAnatomical Therapeutic Chemical (ATC) classificationhttp://www.genome.jp/kegg-bin/get_htext?br08303.kegTarget-based classification of drugshttp://www.genome.jp/kegg-bin/get_htext?br08310.kegDrugs listed in the Japanese Pharmacopoeiahttp://www.genome.jp/kegg-bin/get_htext?br08311.kegRisk category of Japanese OTC drugshttp://www.genome.jp/kegg-bin/get_htext?br08312.kegClassification of Japanese OTC drugshttp://www.genome.jp/kegg-bin/get_htext?br08313.keg
- LIPID MAPSLipid Classificationhttps://www.lipidmaps.org/
- MarkerDBLICENSEThis work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.https://markerdb.ca/Cortisolhttps://markerdb.ca/chemicals/44
- Metabolomics Workbench
- NIPH Clinical Trials Search of Japan
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.htmlhydrocortisonehttps://rxnav.nlm.nih.gov/id/rxnorm/5492
- PharmGKBLICENSEPharmGKB data are subject to the Creative Commons Attribution-ShareALike 4.0 license (https://creativecommons.org/licenses/by-sa/4.0/).https://www.pharmgkb.org/page/policieshydrocortisonehttps://www.pharmgkb.org/chemical/PA449905
- PharosLICENSEData accessed from Pharos and TCRD is publicly available from the primary sources listed above. Please respect their individual licenses regarding proper use and redistribution.https://pharos.nih.gov/abouthydrocortisonehttps://pharos.nih.gov/ligands/62WUFJWYCZNJ
- Protein Data Bank in Europe (PDBe)
- RCSB Protein Data Bank (RCSB PDB)LICENSEData files contained in the PDB archive (ftp://ftp.wwpdb.org) are free of all copyright restrictions and made fully and freely available for both non-commercial and commercial use. Users of the data should attribute the original authors of that structural data.https://www.rcsb.org/pages/policies
- Rhea - Annotated Reactions DatabaseLICENSERhea has chosen to apply the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/). This means that you are free to copy, distribute, display and make commercial use of the database in all legislations, provided you credit (cite) Rhea.https://www.rhea-db.org/help/license-disclaimer
- Springer Nature
- SpringerMaterials
- The Cambridge Structural Database
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- USGS Health-Based Screening Levels for Evaluating Water-Quality DataLICENSEhttps://www.usgs.gov/legalHydrocortisonehttps://water.usgs.gov/water-resources/hbsl/index.html
- Wikidata
- WikipediaHydrocortisonehttps://en.wikipedia.org/wiki/Hydrocortisone
- Wiley
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlHydrocortisonehttps://www.ncbi.nlm.nih.gov/mesh/68006854Anti-Inflammatory Agentshttps://www.ncbi.nlm.nih.gov/mesh/68000893
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403379200https://pubchem.ncbi.nlm.nih.gov/substance/403379200
- NCBI