Isosulfan Blue
- ISOSULFAN BLUE
- 68238-36-8
- Sulphan Blue
- Isosulfan blue [USAN]
- Sulfanblau
- Create:2005-08-08
- Modify:2025-01-18
 isosulfan blue inner salt (has active moiety).
isosulfan blue inner salt (has active moiety).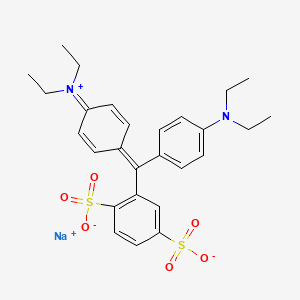
- 4-(alpha-(p-(diethylamino)phenyl)-(2,5-disulfobenzylidene)-2,5-cyclohexadien-1-ylidene)diethylammonium hydroxide inner salt, sodium salt
- iso-sulfan blue
- iso-sulphan blue
- isosulfan blue
- Lymphazurin
- lymphazurin blue
- ISOSULFAN BLUE
- 68238-36-8
- Sulphan Blue
- Isosulfan blue [USAN]
- Sulfanblau
- Disulphin Blau
- Iso-sulfan blue
- Patent blau V
- LYMPHAZURIN
- Sulphan blue 2,5-disulfophenyl isomer
- Sodium 2-((4-(diethylamino)phenyl)(4-(diethyliminio)cyclohexa-2,5-dien-1-ylidene)methyl)benzene-1,4-disulfonate
- UNII-39N9K8S2A4
- DTXSID8057805
- HSDB 8071
- 39N9K8S2A4
- MFCD00867653
- P-4125
- NSC-734902
- DTXCID6031594
- P-1888
- NSC 734902
- Ethanaminium, N-(4-((4-(diethylamino)phenyl)(2,5-disulfophenyl)methylene)-2,5-cyclohexadien-1-ylidene)-N-ethyl-, hydroxide, inner salt, sodium salt
- Isosulfan blue (USAN)
- SODIUM 2-((4-(DIETHYLAMINO)PHENYL)(4-(DIETHYLIMINIO)CYCLOHEXA-2,5-DIEN-1-YLIDENE)METHYL)BENZENE-1,4-DISULPHONATE
- PATENTBLUEVIOLET
- Azul isosulfano
- Bleu isosulfan
- Blu isosulfan
- Azul de isosulfan
- P 1888
- P 4125
- Lymphazurin (TN)
- sodium 2-{[4-(diethylamino)phenyl][4-(diethyliminio)cyclohexa-2,5-dien-1-ylidene]methyl}benzene-1,4-disulfonate
- 2,5-Disulfophenyl isomer
- Isosulfan blue (Standard)
- (4-(alpha-(p-(Diethylamino)phenyl)-2,5-disulfobenzylidene)-2,5-cyclohexadien-1-ylidene)diethylammonium hydroxide, inner salt, sodium salt
- ISOSULFAN BLUE [VANDF]
- Isosulfan blue; Isosulfan blue
- SCHEMBL3324345
- CHEMBL1200859
- ISOSULFAN BLUE [WHO-DD]
- NLUFDZBOHMOBOE-UHFFFAOYSA-M
- Tox21_113733
- ISOSULFAN BLUE [ORANGE BOOK]
- AKOS015904521
- FD10698
- HY-107967R
- NCGC00253597-01
- DA-54462
- sodium;2-[[4-(diethylamino)phenyl]-(4-diethylazaniumylidenecyclohexa-2,5-dien-1-ylidene)methyl]benzene-1,4-disulfonate
- SY238240
- CAS-68238-36-8
- D04634
- SULPHAN BLUE 2,5-DISULFOPHENYL ISOMER [MI]
- (4-(.ALPHA.-(P-(DIETHYLAMINO)PHENYL)-2,5-DISULFOBENZYLIDENE)-2,5-CYCLOHEXADIEN-1-YLIDENE)DIETHYLAMMONIUM HYDROXIDE, INNER SALT, SODIUM SALT
- ETHANAMINIUM, N-(4-((4-(DIETHYLAMINO)PHENYL)(2,5-DISULFOPHENYL)METHYLENE)-2,5-CYCLOHEXADIEN-1-YLIDENE)-N-ETHYL-, INNER SALT, SODIUM SALT
- N-(4-((4-(diethylamino)phenyl)(2,5-disulfophenyl)methylene)-2,5-cyclohexadien-1-ylidene)-N-ethyl-ethanaminium hydroxide
 isosulfan blue inner salt (has active moiety)
isosulfan blue inner salt (has active moiety)
H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation]
H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation]
H335 (100%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation]
P261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Skin Irrit. 2 (100%)
Eye Irrit. 2 (100%)
STOT SE 3 (100%)
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=NLUFDZBOHMOBOE-UHFFFAOYSA-M
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.html
- ChemIDplusIsosulfan blue [USAN]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0068238368ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_useIsosulfan bluehttps://www.drugbank.ca/drugs/DB09136
- EPA DSSToxIsosulfan bluehttps://comptox.epa.gov/dashboard/DTXSID8057805CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticesodium;2-[[4-(diethylamino)phenyl]-(4-diethylazaniumylidenecyclohexa-2,5-dien-1-ylidene)methyl]benzene-1,4-disulfonatehttps://echa.europa.eu/substance-information/-/substanceinfo/100.248.842sodium;2-[[4-(diethylamino)phenyl]-(4-diethylazaniumylidenecyclohexa-2,5-dien-1-ylidene)methyl]benzene-1,4-disulfonate (EC: 814-985-7)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/256418
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Hazardous Substances Data Bank (HSDB)Isosulfan bluehttps://pubchem.ncbi.nlm.nih.gov/source/hsdb/8071
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/Ethanaminium, N-4-4-(diethylamino)phenyl(2,5-disulfophenyl)methylene-2,5-cyclohexadien-1-ylidene-N-ethyl-, inner salt, sodium salthttps://www.epa.govt.nz/industry-areas/hazardous-substances/guidance-for-importers-and-manufacturers/hazardous-substances-databases/
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- DailyMed
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licenceISOSULFAN BLUEhttps://platform.opentargets.org/drug/CHEMBL1200859
- Drugs@FDALICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingISOSULFAN BLUEhttps://www.accessdata.fda.gov/scripts/cder/daf/
- FDA Orange BookLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.html
- Metabolomics Workbench
- National Drug Code (NDC) DirectoryLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.htmliso-sulfan bluehttps://rxnav.nlm.nih.gov/id/rxnorm/27863
- Therapeutic Target Database (TTD)Isosulfan bluehttps://idrblab.net/ttd/data/drug/details/D0P2YD
- Springer Nature
- Wikidataisosulfan blue, sodium salthttps://www.wikidata.org/wiki/Q22075850
- WikipediaThreohydrobupropionhttps://en.wikipedia.org/wiki/ThreohydrobupropionIsosulfan bluehttps://en.wikipedia.org/wiki/Isosulfan_blue
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmliso-sulfan bluehttps://www.ncbi.nlm.nih.gov/mesh/67025484Coloring Agentshttps://www.ncbi.nlm.nih.gov/mesh/68004396
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403791876https://pubchem.ncbi.nlm.nih.gov/substance/403791876
- NCBI
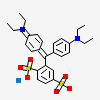

 CID 4695 (4-{[4-(Diethylamino)phenyl](2,5-disulfophenyl)methylidene}-N,N-diethylcyclohexa-2,5-dien-1-iminium)
CID 4695 (4-{[4-(Diethylamino)phenyl](2,5-disulfophenyl)methylidene}-N,N-diethylcyclohexa-2,5-dien-1-iminium) CID 5360545 (Sodium)
CID 5360545 (Sodium)