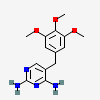
Trimethoprim
- trimethoprim
- 738-70-5
- Proloprim
- Trimpex
- Trimetoprim
- Create:2005-03-25
- Modify:2025-01-18
 Sulfamethoxazole; Trimethoprim (component of);
Sulfamethoxazole; Trimethoprim (component of);  Trimethoprim sulfate (has salt form);
Trimethoprim sulfate (has salt form);  Trimethoprim Hydrochloride (has salt form) ... View More ...
Trimethoprim Hydrochloride (has salt form) ... View More ...
- Proloprim
- Trimethoprim
- Trimpex
- trimethoprim
- 738-70-5
- Proloprim
- Trimpex
- Trimetoprim
- 2,4-Diamino-5-(3,4,5-trimethoxybenzyl)pyrimidine
- Monotrim
- Monotrimin
- Trimopan
- Wellcoprim
- Bactramin
- Monoprim
- Syraprim
- Triprim
- Uretrim
- Trimethoprime
- Trimethoprimum
- 5-(3,4,5-trimethoxybenzyl)pyrimidine-2,4-diamine
- Trimetoprima
- Abaprim
- Briscotrim
- Novotrimel
- Streptoplus
- Sulfoxaprim
- Trimethioprim
- Urobactrim
- Wellcoprin
- Anitrim
- Antrima
- Antrimox
- Bacidal
- Bacticel
- Bactoprim
- Bencole
- Bethaprim
- Biosulten
- Chemotrin
- Colizole
- Conprim
- Cotrimel
- Duocide
- Esbesul
- Espectrin
- Euctrim
- Exbesul
- Fermagex
- Fortrim
- Ikaprim
- Kombinax
- Lagatrim
- Lastrim
- Metoprim
- Pancidim
- Protrin
- Resprim
- Salvatrim
- Setprin
- Sinotrim
- Sugaprim
- Sulfamar
- Sulthrim
- Sultrex
- Trimexol
- Trimezol
- Trimono
- Trisulcom
- Trisulfam
- Trisural
- Utetrin
- Velaten
- Xeroprim
- Zamboprim
- 5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-diamine
- Bacdan
- Bacide
- Bacin
- Bacta
- Deprim
- Futin
- Omstat
- Purbal
- Roubac
- Roubal
- Stopan
- Toprim
- Trisul
- Co-Trimoxizole
- Lagatrim Forte
- component of Bactrim
- Septrin Forte
- Trimpex 200
- Alcorim-F
- Colizole DS
- Septrin S
- Septrin DS
- NSC-106568
- Trimez-IFSA
- U-Prin
- component of Septra
- Dosulfin
- NIH 204
- Resprim Forte
- BW 56-72
- Uro-D S
- Tmp Smx
- Infectotrimet
- Trimethoprime [INN-French]
- Trimethoprimum [INN-Latin]
- Trimetoprima [INN-Spanish]
- 2,4-Pyrimidinediamine, 5-[(3,4,5-trimethoxyphenyl)methyl]-
- Bacterial [Antibiotic]
- CCRIS 2410
- NSC 106568
- WR 5949
- CHEBI:45924
- HSDB 6781
- TCMDC-125538
- BW-56-72
- 5-(3,4,5-Trimethoxybenzyl)-2,4-diaminopyrimidine
- EINECS 212-006-2
- UNII-AN164J8Y0X
- MFCD00036761
- 5-[(3,4,5-Trimethoxyphenyl)methyl]-2,4-pyrimidinediamine
- BRN 0625127
- AN164J8Y0X
- 2,4-Pyrimidinediamine, 5-((3,4,5-trimethoxyphenyl)methyl)-
- 5-(3,4,5-Trimethoxybenzyl)-2,4-pyrimidinediamine
- DTXSID3023712
- AI3-52594
- NIH-204
- Pyrimidine, 2,4-diamino-5-(3,4,5-trimethoxybenzyl)-
- CHEMBL22
- Trimpex (TN)
- NSC106568
- Apo-Sulfatrim
- BW 5672
- MLS000079023
- DTXCID803712
- 5-((3,4,5-Trimethoxyphenyl)methyl)-2,4-pyrimidinediamine
- 2,4-Pyrimidinediamine, 5-((3,4,5-trimethoxyphenyl)-methyl)-
- 5-25-13-00429 (Beilstein Handbook Reference)
- COTRIM COMPONENT TRIMETHOPRIM
- SEPTRA COMPONENT TRIMETHOPRIM
- BACTRIM COMPONENT TRIMETHOPRIM
- Co-trimoxazole component trimethoprim
- UROPLUS COMPONENT TRIMETHOPRIM
- SULFATRIM COMPONENT TRIMETHOPRIM
- SULMEPRIM COMPONENT TRIMETHOPRIM
- BW-5672
- BACTRIM DS COMPONENT TRIMETHOPRIM
- Bacterial (Antibiotic)
- NCGC00016055-05
- Trimethoprim [USAN:USP:INN:BAN:JAN]
- Trimethopriom
- Bactifor
- CAS-738-70-5
- Instalac
- SMR000035999
- Trimetoprim [DCIT]
- Trimogal
- COTRIM D.S. COMPONENT TRIMETHOPRIM
- Lescot
- Tiempe
- Trimetoprim [Polish]
- SULFAMETHOPRIM COMPONENT TRIMETHOPRIM
- Trimethoprim 100 microg/mL in Acetonitrile
- BACTRIM PEDIATRIC COMPONENT TRIMETHOPRIM
- Trimethoprime (INN-French)
- Trimethoprimum (INN-Latin)
- Trimetoprima (INN-Spanish)
- TRIMETHOPRIM (MART.)
- TRIMETHOPRIM [MART.]
- TRIMETHOPRIM (USP-RS)
- TRIMETHOPRIM [USP-RS]
- 2,4-Diamino-5-(3',4',5'-trimethoxybenzyl)pyrimidine
- TRIMETHOPRIM (EP IMPURITY)
- TRIMETHOPRIM [EP IMPURITY]
- TRIMETHOPRIM (EP MONOGRAPH)
- TRIMETHOPRIM [EP MONOGRAPH]
- NIH 204 (VAN)
- TRIMETHOPRIM (USP MONOGRAPH)
- TRIMETHOPRIM [USP MONOGRAPH]
- Proloprim (TN)
- Trimethoprim (USAN:USP:INN:BAN:JAN)
- Trimethoprim D3 (4-methoxy D3)
- SR-01000075652
- 5-(3, 4, 5-Trimethoxybenzyl)-2, 4-pyrimidinediamine
- Trimethoprim & VRC3375
- Trimethoprim (JAN/USP/INN)
- 5-{[3,4,5-tris(methyloxy)phenyl]methyl}pyrimidine-2,4-diamine
- B-Lock
- KUC103659N
- Trimethoprim,(S)
- Prestwick_485
- KSC-4-158
- Trimethoprim (TMP)
- Bactrim (Salt/Mix)
- Spectrum_000167
- Tocris-0650
- 2w9h
- 3fl9
- 3n0h
- 3s3v
- 4km2
- Trimethoprim (Standard)
- Trimethoprim D3 (4-methoxy D3) 100 microg/mL in Acetonitrile
- Opera_ID_1760
- Prestwick0_000208
- Prestwick1_000208
- Prestwick2_000208
- Prestwick3_000208
- Spectrum2_000937
- Spectrum3_000643
- Spectrum4_000372
- Spectrum5_001559
- Lopac-T-7883
- TRIMETHOPRIM [MI]
- TRIMETHOPRIM [INN]
- TRIMETHOPRIM [JAN]
- Epitope ID:119684
- UPCMLD-DP132
- T 7883
- TRIMETHOPRIM [HSDB]
- TRIMETHOPRIM [USAN]
- TRIMETHOPRIM [VANDF]
- Lopac0_001271
- Oprea1_495058
- SCHEMBL24506
- BSPBio_000195
- BSPBio_002245
- KBioGR_000863
- KBioSS_000647
- MLS001201740
- MLS002303068
- MLS002548881
- BIDD:GT0190
- DivK1c_000589
- SPECTRUM1500595
- TRIMETHOPRIM [WHO-DD]
- SPBio_000874
- SPBio_002116
- BPBio1_000215
- UPCMLD-DP132:001
- BDBM18069
- GTPL10931
- HMS501N11
- HY-B0510R
- KBio1_000589
- KBio2_000647
- KBio2_003215
- KBio2_005783
- KBio3_001465
- J01EA01
- TRIMETHOPRIM [GREEN BOOK]
- Trimethoprim, >=98% (HPLC)
- NINDS_000589
- 2,4,5-trimethoxybenzyl)pyrimidine
- HMS1568J17
- HMS1921I03
- HMS2090D14
- HMS2092A10
- HMS2095J17
- HMS2230L06
- HMS3259I11
- HMS3263P04
- HMS3371O18
- HMS3652E03
- HMS3712J17
- Pharmakon1600-01500595
- TRIMETHOPRIM [ORANGE BOOK]
- Trimethoprim for system suitability
- 2,4,5-trimethoxyphenzyl)pyrimidine
- ALBB-028968
- BCP12148
- HY-B0510
- Tox21_110291
- Tox21_200157
- Tox21_501271
- BBL005584
- CCG-40335
- NSC752719
- NSC757370
- s3129
- STK177322
- STL455117
- TRIMETHOPRIMUM [WHO-IP LATIN]
- AKOS001650069
- Tox21_110291_1
- 1ST4009
- AC-8427
- DB00440
- KS-1145
- LP01271
- NC00483
- NSC-752719
- NSC-757370
- SDCCGSBI-0051237.P004
- TRIMETHOPRIM COMPONENT OF COTRIM
- TRIMETHOPRIM COMPONENT OF SEPTRA
- IDI1_000589
- SMP2_000262
- TRIMETHOPRIM COMPONENT OF BACTRIM
- TRIMETHOPRIM COMPONENT OF UROPLUS
- NCGC00016055-01
- NCGC00016055-02
- NCGC00016055-03
- NCGC00016055-04
- NCGC00016055-06
- NCGC00016055-07
- NCGC00016055-08
- NCGC00016055-09
- NCGC00016055-10
- NCGC00016055-11
- NCGC00016055-12
- NCGC00016055-13
- NCGC00016055-14
- NCGC00016055-16
- NCGC00016055-17
- NCGC00016055-27
- NCGC00024707-01
- NCGC00024707-03
- NCGC00024707-04
- NCGC00024707-05
- NCGC00024707-06
- NCGC00024707-07
- NCGC00024707-08
- NCGC00257711-01
- NCGC00261956-01
- SY031734
- TRIMETHOPRIM COMPONENT OF SULFATRIM
- TRIMETHOPRIM COMPONENT OF SULMEPRIM
- Trimethoprim/sulfamethoxazole (commercial)
- SBI-0051237.P003
- DB-055812
- TRIMETHOPRIM COMPONENT OF BACTRIM DS
- 2, 5-[(3,4,5-trimethoxyphenyl)methyl]-
- AB00052118
- EU-0101271
- NS00000211
- SW196690-3
- T2286
- Trimethoprim 1000 microg/mL in Acetonitrile
- TRIMETHOPRIM COMPONENT OF COTRIM D.S.
- C01965
- D00145
- EN300-118703
- TRIMETHOPRIM COMPONENT OF SULFAMETHOPRIM
- Trimethoprim, crystallized, >=99.0% (HPLC)
- WLN: T6N CNJ BZ DZ E1R CO1 DO1 EO1
- 5-(3,5-Trimethoxybenzyl)-2,4-diaminopyrimidine
- AB00052118-30
- AB00052118-32
- AB00052118_33
- AB00052118_34
- Trimethoprim, VETRANAL(TM), analytical standard
- Q422665
- TRIMETHOPRIM COMPONENT OF BACTRIM PEDIATRIC
- 2,4-diamino-5-(3,4,5-trimethoxybenzyl)-pyrimidine
- Pyrimidine,4-diamino-5-(3,4,5-trimethoxybenzyl)-
- SR-01000075652-1
- SR-01000075652-3
- SR-01000075652-6
- W-104441
- 5-(3,4,5-Trimethoxybenzyl)-2,4-pyrimidinediamine #
- BRD-K07208025-001-06-5
- BRD-K07208025-001-29-7
- BRD-K07208025-001-31-3
- SR-01000075652-10
- F0914-5266
- Trimethoprim, certified reference material, TraceCERT(R)
- Z1515385071
- 5-(3,4,5-trimethoxybenzyl)pyrimidine-2,4(1H,3H)-diimine
- 5-(3,4,5-Trimethoxyphenyl)methyl)-2,4-pyrimidinediamine
- 5-(3,4,5-TRIMETHYOXYBENZYL)-2,4-DIAMINOPYRIMIDINE
- pyrimidine, 2,4-diamino-5-(3',4',5'-trimethoxybenzyl)-
- Trimethoprim, British Pharmacopoeia (BP) Reference Standard
- Trimethoprim, European Pharmacopoeia (EP) Reference Standard
- 5-((3,4,5-TRIMETHOXYPHENYL)-METHYL-2,4-PYRIMIDINEDIAMINE
- Trimethoprim, United States Pharmacopeia (USP) Reference Standard
- Trimethoprim for system suitability, European Pharmacopoeia (EP) Reference Standard
- Trimethoprim, Pharmaceutical Secondary Standard; Certified Reference Material
169.82 Ų [M+Na]+ [CCS Type: TW; Method: calibrated with polyalanine and drug standards]
165.75 Ų [M+K]+ [CCS Type: TW; Method: calibrated with polyalanine and drug standards]
169.64 Ų [M+H]+ [CCS Type: TW; Method: calibrated with polyalanine and drug standards]
158.24 Ų [M+H-H2O]+ [CCS Type: TW; Method: calibrated with polyalanine and drug standards]
172.89 Ų [M+H]+
187.42 Ų [M+Na]+
290.0 99.99
259.0 25.91
275.0 22.41
43.0 21.09
28.0 19.20
290 99.99
259 25.91
275 22.41
43 21.09
28 19.20
123.0666 100
81.0448 47.43
229.1085 33.04
233.1034 26.72
232.0956 26.36
230.1166 100
123.0667 63.80
261.0985 44.31
258.1113 38.99
275.1141 24.62
291.14633 999
123.06696 146
261.099 72
229.10901 71
257.10446 62
289.13138 999
227.05772 442
274.10693 252
243.05194 136
198.0545 62
291.14627 999
123.06676 183
261.09897 59
81.04478 58
257.10388 52
290 999
259 259
275 224
43 211
28 192
Sulfamethoxazole; Trimethoprim (component of)
Trimethoprim sulfate (has salt form)
Trimethoprim Hydrochloride (has salt form)
Trimethoprim lactate (is active moiety of)
- Sulfadiazine; Trimethoprim (component of)
- Sulfadiazine Sodium; Trimethoprim (component of)
- Phenazopyridine hydrochloride; sulfamethoxazole; trimethoprim (component of)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01E - Sulfonamides and trimethoprim
J01EA - Trimethoprim and derivatives
J01EA01 - Trimethoprim
Use (kg) in Switzerland (2009): >250
Use (kg; approx.) in Germany (2009): ?7500
Use (kg; exact) in Germany (2009): 7475
Use (kg) in USA (2002): 463
Use (kg) in France (2004): 3346
Consumption (g per capita) in Switzerland (2009): 0.032
Consumption (g per capita; approx.) in Germany (2009): 0.092
Consumption (g per capita; exact) in Germany (2009): 0.091
Consumption (g per capita) in the USA (2002): 0.0016
Consumption (g per capita) in France (2004): 0.055
Excretion rate: 0.6
Calculated removal (%): 8.8



H302 (85%): Harmful if swallowed [Warning Acute toxicity, oral]
H361 (30.7%): Suspected of damaging fertility or the unborn child [Warning Reproductive toxicity]
H372 (26.4%): Causes damage to organs through prolonged or repeated exposure [Danger Specific target organ toxicity, repeated exposure]
H411 (26.4%): Toxic to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard]
P203, P260, P264, P270, P273, P280, P301+P317, P318, P319, P330, P391, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 140 reports by companies from 21 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Reported as not meeting GHS hazard criteria per 11 of 140 reports by companies. For more detailed information, please visit ECHA C&L website.
There are 20 notifications provided by 129 of 140 reports by companies with hazard statement code(s).
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Acute Tox. 4 (85%)
Repr. 2 (30.7%)
STOT RE 1 (26.4%)
Aquatic Chronic 2 (26.4%)
Acute toxicity (Oral) - Category 3
Reproductive toxicity - Category 1A, Additional category: Effects on or via lactation
Specific target organ toxicity - Repeated exposure - Category 2 (skin, hematopoietic system)
Hazardous to the aquatic environment (Acute) - Category 2
Hazardous to the aquatic environment (Long-term) - Category 2
SYMPTOMS: Symptoms of exposure to this compound may include rash, pruritis, dermatitis, epigastric distress, nausea, and vomiting.
ACUTE/CHRONIC HAZARDS: This material may cause skin irritation and rash. (NTP, 1992)
EYES: First check the victim for contact lenses and remove if present. Flush victim's eyes with water or normal saline solution for 20 to 30 minutes while simultaneously calling a hospital or poison control center. Do not put any ointments, oils, or medication in the victim's eyes without specific instructions from a physician. IMMEDIATELY transport the victim after flushing eyes to a hospital even if no symptoms (such as redness or irritation) develop.
SKIN: IMMEDIATELY flood affected skin with water while removing and isolating all contaminated clothing. Gently wash all affected skin areas thoroughly with soap and water. If symptoms such as redness or irritation develop, IMMEDIATELY call a physician and be prepared to transport the victim to a hospital for treatment.
INHALATION: IMMEDIATELY leave the contaminated area; take deep breaths of fresh air. If symptoms (such as wheezing, coughing, shortness of breath, or burning in the mouth, throat, or chest) develop, call a physician and be prepared to transport the victim to a hospital. Provide proper respiratory protection to rescuers entering an unknown atmosphere. Whenever possible, Self-Contained Breathing Apparatus (SCBA) should be used; if not available, use a level of protection greater than or equal to that advised under Protective Clothing.
INGESTION: DO NOT INDUCE VOMITING. If the victim is conscious and not convulsing, give 1 or 2 glasses of water to dilute the chemical and IMMEDIATELY call a hospital or poison control center. Be prepared to transport the victim to a hospital if advised by a physician. If the victim is convulsing or unconscious, do not give anything by mouth, ensure that the victim's airway is open and lay the victim on his/her side with the head lower than the body. DO NOT INDUCE VOMITING. IMMEDIATELY transport the victim to a hospital. (NTP, 1992)
Excerpt from ERG Guide 154 [Substances - Toxic and/or Corrosive (Non-Combustible)]:
IMMEDIATE PRECAUTIONARY MEASURE: Isolate spill or leak area in all directions for at least 50 meters (150 feet) for liquids and at least 25 meters (75 feet) for solids.
SPILL: Increase the immediate precautionary measure distance, in the downwind direction, as necessary.
FIRE: If tank, rail tank car or highway tank is involved in a fire, ISOLATE for 800 meters (1/2 mile) in all directions; also, consider initial evacuation for 800 meters (1/2 mile) in all directions. (ERG, 2024)
SMALL SPILLS AND LEAKAGE: If a spill of this chemical occurs, FIRST REMOVE ALL SOURCES OF IGNITION, then you should dampen the solid spill material with acetone and transfer the dampened material to a suitable container. Use absorbent paper dampened with acetone to pick up any remaining material. Seal your contaminated clothing and the absorbent paper in a vapor-tight plastic bag for eventual disposal. Solvent wash all contaminated surfaces with acetone followed by washing with a soap and water solution. Do not reenter the contaminated area until the Safety Officer (or other responsible person) has verified that the area has been properly cleaned.
STORAGE PRECAUTIONS: You should store this chemical under refrigerated temperatures, and keep it away from oxidizing materials. (NTP, 1992)
Amines, Phosphines, and Pyridines
Ethers
M Chen, V Vijay, Q Shi, Z Liu, H Fang, W Tong. FDA-Approved Drug Labeling for the Study of Drug-Induced Liver Injury, Drug Discovery Today, 16(15-16):697-703, 2011. PMID:21624500 DOI:10.1016/j.drudis.2011.05.007
M Chen, A Suzuki, S Thakkar, K Yu, C Hu, W Tong. DILIrank: the largest reference drug list ranked by the risk for developing drug-induced liver injury in humans. Drug Discov Today 2016, 21(4): 648-653. PMID:26948801 DOI:10.1016/j.drudis.2016.02.015
◉ Summary of Use during Lactation
Because of the low levels of trimethoprim in breastmilk, amounts ingested by the infant are small and would not be expected to cause any adverse effects in breastfed infants.
◉ Effects in Breastfed Infants
In one study, no adverse effects were noted in infants during 4 days of maternal therapy with co-trimoxazole.
In a telephone follow-up study, 12 nursing mothers reported taking co-trimoxazole (dosage unspecified). Two mothers reported poor feeding in their infants. Diarrhea was not reported among the exposed infants.
◉ Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=IEDVJHCEMCRBQM-UHFFFAOYSA-N
- Australian Industrial Chemicals Introduction Scheme (AICIS)2,4-Pyrimidinediamine, 5-[(3,4,5-trimethoxyphenyl)methyl]-https://services.industrialchemicals.gov.au/search-inventory/
- CAMEO ChemicalsLICENSECAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data.https://cameochemicals.noaa.gov/help/reference/terms_and_conditions.htm?d_f=falseTRIMETHOPRIMhttps://cameochemicals.noaa.gov/chemical/21180CAMEO Chemical Reactivity Classificationhttps://cameochemicals.noaa.gov/browse/react
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusTrimethoprim [USAN:USP:INN:BAN:JAN]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000738705ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_useTrimethoprimhttps://www.drugbank.ca/drugs/DB00440
- DTP/NCILICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reusetrimethoprimhttps://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=757370
- EPA DSSToxTrimethoprimhttps://comptox.epa.gov/dashboard/DTXSID3023712CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeTrimethoprim (EC: 212-006-2)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/121093
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Hazardous Substances Data Bank (HSDB)
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingTrimethoprimhttp://www.hmdb.ca/metabolites/HMDB0014583HMDB0014583_cms_29724https://hmdb.ca/metabolites/HMDB0014583#spectra
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/
- BindingDBLICENSEAll data curated by BindingDB staff are provided under the Creative Commons Attribution 3.0 License (https://creativecommons.org/licenses/by/3.0/us/).https://www.bindingdb.org/rwd/bind/info.jsp5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-diaminehttps://www.bindingdb.org/rwd/bind/chemsearch/marvin/MolStructure.jsp?monomerid=18069
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jsp
- Therapeutic Target Database (TTD)Trimethoprimhttps://idrblab.net/ttd/data/drug/details/D0AO5H
- ChEBI
- FDA Pharm ClassesLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingFDA Pharmacological Classificationhttps://www.fda.gov/ForIndustry/DataStandards/StructuredProductLabeling/ucm162549.htm
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licenceTRIMETHOPRIMhttps://platform.opentargets.org/drug/CHEMBL22
- CCSbaseCCSbase Classificationhttps://ccsbase.net/
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/TRIMETHOPRIMNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- Crystallography Open Database (COD)LICENSEAll data in the COD and the database itself are dedicated to the public domain and licensed under the CC0 License. Users of the data should acknowledge the original authors of the structural data.https://creativecommons.org/publicdomain/zero/1.0/
- The Cambridge Structural Database
- DailyMed
- Drug Induced Liver Injury Rank (DILIrank) DatasetLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- IUPAC Digitized pKa Datasetpyrimidine, 2,4-diamino-5-(3',4',5'-trimethoxybenzyl)-https://github.com/IUPAC/Dissociation-Constants
- Drugs and Lactation Database (LactMed)
- Drugs@FDALICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- WHO Model Lists of Essential MedicinesLICENSEPermission from WHO is not required for the use of WHO materials issued under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Intergovernmental Organization (CC BY-NC-SA 3.0 IGO) license.https://www.who.int/about/policies/publishing/copyrightTrimethoprimhttps://list.essentialmeds.org/medicines/599
- EPA Chemical and Products Database (CPDat)EPA CPDat Classificationhttps://www.epa.gov/chemical-research/chemical-and-products-database-cpdat
- EU Clinical Trials Register
- NITE-CMC2,4-Diamino-5-(3,4,5-trimethoxybenzyl)pyrimidine [Trimethoprim] - FY2017 (New/original classication)https://www.chem-info.nite.go.jp/chem/english/ghs/17-mhlw-0016e.html
- FDA Approved Animal Drug Products (Green Book)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- FDA Orange BookLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- National Drug Code (NDC) DirectoryLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license2,4-DIAMINO-5-(3,4,5-TRIMETHOXYBENZYL)PYR-IMIDINEhttps://mona.fiehnlab.ucdavis.edu/spectra/browse?query=exists(compound.metaData.name:%27InChIKey%27%20and%20compound.metaData.value:%27IEDVJHCEMCRBQM-UHFFFAOYSA-N%27)
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawTrimethoprimhttp://www.nist.gov/srd/nist1a.cfm
- SpectraBaseTrimethoprimhttps://spectrabase.com/spectrum/Jxx16CZxnIzTrimethoprimhttps://spectrabase.com/spectrum/GDYDp4POsXnTrimethoprimhttps://spectrabase.com/spectrum/JpgQLEIHP162,4-DIAMINO-5-(3,4,5-TRIMETHOXYBENZYL)-PYRIMIDINEhttps://spectrabase.com/spectrum/lfor97OAy12,4-DIAMINO-5-(3,4,5-TRIMETHOXYBENZYL)-PYRIMIDINEhttps://spectrabase.com/spectrum/Gx8XgBLlmw92,4-DIAMINO-5-(3,4,5-TRIMETHOXYBENZYL)PYRIMIDINEhttps://spectrabase.com/spectrum/Dyhc4SPHbbk2,4-diamino-5-(3,4,5-trimethoxybenzyl)pyrimidinehttps://spectrabase.com/spectrum/4pdY6uYrm2c2,4-diamino-5-(3,4,5-trimethoxybenzyl)pyrimidinehttps://spectrabase.com/spectrum/EHyY9BkzGOqTrimethoprimhttps://spectrabase.com/spectrum/98NGU6DsEZeTrimethoprimhttps://spectrabase.com/spectrum/7Ythnv7x9TN
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlUSP drug classificationhttp://www.genome.jp/kegg-bin/get_htext?br08302.kegAnatomical Therapeutic Chemical (ATC) classificationhttp://www.genome.jp/kegg-bin/get_htext?br08303.kegAntiinfectiveshttp://www.genome.jp/kegg-bin/get_htext?br08307.kegAnimal drugs in Japanhttp://www.genome.jp/kegg-bin/get_htext?br08331.keg
- MassBank Europe
- Metabolomics Workbench
- Nature Chemical Biology
- NIPH Clinical Trials Search of Japan
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.htmltrimethoprimhttps://rxnav.nlm.nih.gov/id/rxnorm/10829
- WHO Anatomical Therapeutic Chemical (ATC) ClassificationLICENSEUse of all or parts of the material requires reference to the WHO Collaborating Centre for Drug Statistics Methodology. Copying and distribution for commercial purposes is not allowed. Changing or manipulating the material is not allowed.https://www.whocc.no/copyright_disclaimer/Trimethoprimhttps://www.whocc.no/atc_ddd_index/?code=J01EA01
- PharmGKBLICENSEPharmGKB data are subject to the Creative Commons Attribution-ShareALike 4.0 license (https://creativecommons.org/licenses/by-sa/4.0/).https://www.pharmgkb.org/page/policiestrimethoprimhttps://www.pharmgkb.org/chemical/PA451788
- PharosLICENSEData accessed from Pharos and TCRD is publicly available from the primary sources listed above. Please respect their individual licenses regarding proper use and redistribution.https://pharos.nih.gov/aboutTrimethoprimhttps://pharos.nih.gov/ligands/1UYSPXCG15AL
- Protein Data Bank in Europe (PDBe)
- RCSB Protein Data Bank (RCSB PDB)LICENSEData files contained in the PDB archive (ftp://ftp.wwpdb.org) are free of all copyright restrictions and made fully and freely available for both non-commercial and commercial use. Users of the data should attribute the original authors of that structural data.https://www.rcsb.org/pages/policies
- Springer Nature
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- USGS Health-Based Screening Levels for Evaluating Water-Quality DataLICENSEhttps://www.usgs.gov/legal
- Wikidatatrimethoprimhttps://www.wikidata.org/wiki/Q422665
- Wikipediatrimethoprimhttps://en.wikipedia.org/wiki/Trimethoprim
- Wiley
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlTrimethoprimhttps://www.ncbi.nlm.nih.gov/mesh/68014295Cytochrome P-450 CYP2C8 Inhibitorshttps://www.ncbi.nlm.nih.gov/mesh/68065687Anti-Dyskinesia Agentshttps://www.ncbi.nlm.nih.gov/mesh/68018726Antimalarialshttps://www.ncbi.nlm.nih.gov/mesh/68000962Anti-Infective Agents, Urinaryhttps://www.ncbi.nlm.nih.gov/mesh/68000892Folic Acid Antagonistshttps://www.ncbi.nlm.nih.gov/mesh/68005493
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403034161https://pubchem.ncbi.nlm.nih.gov/substance/403034161
- NCBI