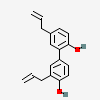
Honokiol
- Honokiol
- 35354-74-6
- 5,3'-Diallyl-2,4'-dihydroxybiphenyl
- NSC 293100
- 3,5'-Diallyl-4,2'-dihydroxybiphenyl
- Create:2005-03-26
- Modify:2025-01-04
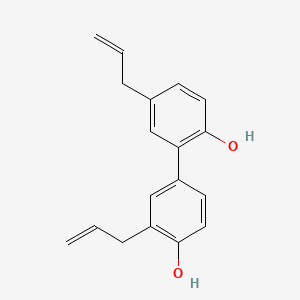
- (1,1'-biphenyl)-2,4'-diol, 3',5-di-2-propen-1-yl-
- 3,5'-diallyl-4,2'-dihydroxybiphenyl
- honokiol
- Honokiol
- 35354-74-6
- 5,3'-Diallyl-2,4'-dihydroxybiphenyl
- NSC 293100
- 3,5'-Diallyl-4,2'-dihydroxybiphenyl
- 3',5-Diallylbiphenyl-2,4'-diol
- C18H18O2
- 2-(4-hydroxy-3-prop-2-enylphenyl)-4-prop-2-enylphenol
- MFCD00016674
- CPD000387107
- 3',5-Di-2-propen-1-yl[1,1'-biphenyl]-2,4'-diol
- CHEMBL16901
- CHEBI:5759
- 4-allyl-2-(3-allyl-4-hydroxy-phenyl)phenol
- 11513CCO0N
- 2-[4-hydroxy-3-(prop-2-en-1-yl)phenyl]-4-(prop-2-en-1-yl)phenol
- NSC293100
- [1,1'-Biphenyl]-2,4'-diol, 3',5-di-2-propenyl-
- NSC-293100
- Honokiol,(S)
- 2-(4-hydroxy-3-prop-2-enyl-phenyl)- 4-prop-2-enyl-phenol
- (1P)-3',5-di(prop-2-en-1-yl)[1,1'-biphenyl]-2,4'-diol
- 3',5-(5,5'-)Diallyl-[1,1'-biphenyl]-2,4'-(2,2'-)diol
- SMR000387107
- 3',5-di(prop-2-en-1-yl)biphenyl-2,4'-diol
- 3',5-diallyl-2,4'-biphenyldiol
- Purinol
- (1,1'-BIPHENYL)-2,4'-DIOL, 3',5-DI-2-PROPENYL-
- UNII-11513CCO0N
- Honokiol?
- -dihydroxydiphenyl
- 3',5-diallyl-[1,1'-biphenyl]-2,4'-diol
- Honokiol, HO
- 3',5-Di-2-propen-1-yl-[1,1'-biphenyl]-2,4'-diol; [1,1'-Biphenyl]-2,4'-diol, 3',5-di-2-propenyl- (9CI); 3',5-Diallyl-2,4'-biphenyldiol; Honokiol; NSC 293100
- Y4T
- Honokiol (Standard)
- 5,3&prime
- Honokiol - 95%
- -Diallyl-2,4&prime
- FMLT BSASM H
- HONOKIOL [MI]
- HONOKIOL [WHO-DD]
- Honokiol, analytical standard
- cid_72303
- MLS000759481
- MLS001048916
- MLS001423980
- MLS006011755
- SCHEMBL133034
- REGID_for_CID_72303
- GTPL11610
- HY-N0003R
- DTXSID30188845
- 3',1'-biphenyl)-2,4'-diol
- HMS2051C12
- HMS2271J07
- HMS3393C12
- HMS3656G03
- (1,1'-BIPHENYL)-2,4'-DIOL, 3',5-DI-2-PROPEN-1-YL-
- BCP28282
- HY-N0003
- 5,3'-Diallyl-biphenyl-2,4'-diol
- AC-486
- BBL027819
- BDBM50157304
- HB0328
- s2310
- STK801954
- 3'',5-diallylbiphenyl-2,4''-diol
- Honokiol, >=98% (HPLC), powder
- 3,5'-diallyl-2',4-dihydroxybiphenyl
- AKOS005622639
- 3',5-Diallyl-2,4'-dihydroxybiphenyl
- CCG-100864
- CS-1696
- NC00114
- 5,3''-Diallyl-biphenyl-2,4''-diol
- SMP2_000040
- NCGC00163567-01
- NCGC00163567-02
- NCGC00163567-03
- NCGC00163567-08
- AS-15333
- XH163752
- 5,3''''-Diallyl-biphenyl-2,4''''-diol
- H1309
- NS00011726
- SW197494-3
- EN300-7399522
- SR-01000758208
- Q-100425
- Q5896650
- SR-01000758208-5
- {1,1'-Biphenyl]-2,4'-diol, 3,5-di-2-propenyl-
- 3'',5-di-2-propenyl-1,1''-biphenyl-2,4''-diol
- BRD-K98493452-001-01-6
- BRD-K98493452-001-14-9
- Honokiol, European Pharmacopoeia (EP) Reference Standard
- Z2065671480
- [1,1'-Biphenyl]-2,4'-diol, 3',5-di-2-propen-1-yl
- 4-[2-hydroxy-5-(prop-2-en-1-yl)phenyl]-2-(prop-2-en-1-yl)phenol
- InChI=1/C18H18O2/c1-3-5-13-7-9-18(20)16(11-13)14-8-10-17(19)15(12-14)6-4-2/h3-4,7-12,19-20H,1-2,5-6H
- N-[[4-([1,2,4]Triazolo[1,5-a]pyridin-6-yl)-5-(6-methylpyridin-2-yl)-1H-imidazol-2-yl]methyl]-2-fluoroaniline; N-?(2-?Fluorophenyl)?-?5-?(6-?methyl-?2-?pyridinyl)?-?4-?[1,?2,?4]?triazolo[1,?5-?a]?pyridin-?6-?yl-1H-?imidazole-?2-?methanamine
267.14139 100
266.13123 33.50
226.09779 33.50
197.05568 24.10
225.08344 21.20
267.13724 100
266.12732 70
239.10231 25.40
226.09184 16.20
251.10594 4.30
266.13205 999
239.10782 823
267.14154 500
267.12967 288
197.06303 134
267.13867 999
266.13089 408
239.1058 112
211.07742 104
227.10393 97



H302 (28%): Harmful if swallowed [Warning Acute toxicity, oral]
H315 (28%): Causes skin irritation [Warning Skin corrosion/irritation]
H318 (47%): Causes serious eye damage [Danger Serious eye damage/eye irritation]
H319 (53%): Causes serious eye irritation [Warning Serious eye damage/eye irritation]
H335 (28%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation]
H411 (36%): Toxic to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard]
P261, P264, P264+P265, P270, P271, P273, P280, P301+P317, P302+P352, P304+P340, P305+P351+P338, P305+P354+P338, P317, P319, P321, P330, P332+P317, P337+P317, P362+P364, P391, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 100 reports by companies from 5 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Acute Tox. 4 (28%)
Skin Irrit. 2 (28%)
Eye Dam. 1 (47%)
Eye Irrit. 2 (53%)
STOT SE 3 (28%)
Aquatic Chronic 2 (36%)
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=FVYXIJYOAGAUQK-UHFFFAOYSA-N
- BindingDBLICENSEAll data curated by BindingDB staff are provided under the Creative Commons Attribution 3.0 License (https://creativecommons.org/licenses/by/3.0/us/).https://www.bindingdb.org/rwd/bind/info.jsp3',5-di-2-propenyl-1,1'-biphenyl-2,4'-diolhttps://www.bindingdb.org/rwd/bind/chemsearch/marvin/MolStructure.jsp?monomerid=50157304
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jsp
- IUPHAR/BPS Guide to PHARMACOLOGYLICENSEThe Guide to PHARMACOLOGY database is licensed under the Open Data Commons Open Database License (ODbL) https://opendatacommons.org/licenses/odbl/. Its contents are licensed under a Creative Commons Attribution-ShareAlike 4.0 International License (http://creativecommons.org/licenses/by-sa/4.0/)https://www.guidetopharmacology.org/about.jsp#licenseGuide to Pharmacology Target Classificationhttps://www.guidetopharmacology.org/targets.jsp
- Therapeutic Target Database (TTD)
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DTP/NCILICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuse
- EPA DSSToxCompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeHonokiol (EC: 609-119-8)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/78823
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/
- ChEBI
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/LOTUS Treehttps://lotus.naturalproducts.net/
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- Crystallography Open Database (COD)LICENSEAll data in the COD and the database itself are dedicated to the public domain and licensed under the CC0 License. Users of the data should acknowledge the original authors of the structural data.https://creativecommons.org/publicdomain/zero/1.0/
- The Cambridge Structural Database
- EPA Chemical and Products Database (CPDat)EPA CPDat Classificationhttps://www.epa.gov/chemical-research/chemical-and-products-database-cpdat
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingHMDB0253212_msms_2226599https://hmdb.ca/metabolites/HMDB0253212#spectra
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlPhytochemical compoundshttp://www.genome.jp/kegg-bin/get_htext?br08003.keg
- KNApSAcK Species-Metabolite Database
- Natural Product Activity and Species Source (NPASS)
- MassBank Europe
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- Metabolomics Workbench
- Nature Chemical Biology
- NMRShiftDB
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/HonokiolNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- PharosLICENSEData accessed from Pharos and TCRD is publicly available from the primary sources listed above. Please respect their individual licenses regarding proper use and redistribution.https://pharos.nih.gov/about
- Protein Data Bank in Europe (PDBe)
- RCSB Protein Data Bank (RCSB PDB)LICENSEData files contained in the PDB archive (ftp://ftp.wwpdb.org) are free of all copyright restrictions and made fully and freely available for both non-commercial and commercial use. Users of the data should attribute the original authors of that structural data.https://www.rcsb.org/pages/policies
- SpectraBaseHONOKIOL;(1,1'-BIPHENYL)-2,4'-DIOL-3'-(1E)-1-PROPEN-1-YL-5-(2-PROPEN-1-YL)https://spectrabase.com/spectrum/ArRpYG0bIbY
- Springer Nature
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidata
- Wikipedia
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlAnti-Anxiety Agentshttps://www.ncbi.nlm.nih.gov/mesh/68014151Anti-Arrhythmia Agentshttps://www.ncbi.nlm.nih.gov/mesh/68000889Central Nervous System Depressantshttps://www.ncbi.nlm.nih.gov/mesh/68002492Anti-Allergic Agentshttps://www.ncbi.nlm.nih.gov/mesh/68018926Antineoplastic Agents, Phytogenichttps://www.ncbi.nlm.nih.gov/mesh/68000972Enzyme Inhibitorshttps://www.ncbi.nlm.nih.gov/mesh/68004791Gastrointestinal Agentshttps://www.ncbi.nlm.nih.gov/mesh/68005765Anti-Infective Agentshttps://www.ncbi.nlm.nih.gov/mesh/68000890
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 388519629https://pubchem.ncbi.nlm.nih.gov/substance/388519629
- NCBI