Histamine Dihydrochloride
- C5H9N3.2ClH
- C5H11Cl2N3
- Histamine dihydrochloride
- 56-92-8
- Histamine 2HCl
- 2-(1H-Imidazol-4-yl)ethanamine dihydrochloride
- 1H-Imidazole-4-ethanamine dihydrochloride
- Create:2005-03-27
- Modify:2024-12-27
 Histamine (has active moiety); Histamine dihydrochloride; menthol (component of); Capsaicin; histamine dihydrochloride; menthol (component of) ... View More ...
Histamine (has active moiety); Histamine dihydrochloride; menthol (component of); Capsaicin; histamine dihydrochloride; menthol (component of) ... View More ...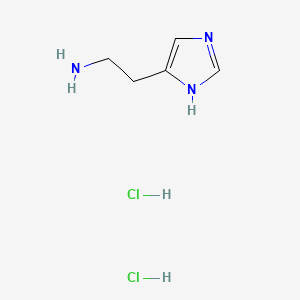
- Ceplene
- Histamine
- Histamine Dihydrochloride
- Histamine Hydrochloride
- Peremin
- Histamine dihydrochloride
- 56-92-8
- Histamine 2HCl
- 2-(1H-Imidazol-4-yl)ethanamine dihydrochloride
- 1H-Imidazole-4-ethanamine dihydrochloride
- peremin
- 2-(4-Imidazolyl)ethylamine dihydrochloride
- Ceplene
- 2-(1H-Imidazol-4-yl)ethylamine dihydrochloride
- 1H-Imidazole-4-ethanamine, dihydrochloride
- Histaminedium dichloride
- Histamine dichloride
- Histaminedihydrochloride
- MFCD00012703
- Histamine chloride
- histaminum hydrochloricum
- HISTAMINE, DIHYDROCHLORIDE
- Histamine dihydrochloride [USAN]
- 4-(2-Aminoethyl)imidazole dihydrochloride
- 2-(1H-imidazol-4-yl)ethan-1-amine dihydrochloride
- EINECS 200-298-4
- UNII-3POA0Q644U
- NSC 257873
- 3POA0Q644U
- 2-Imidazol-4-ylethylamine dihydrochloride
- Histamine HCl
- AI3-24394
- histamine dichlorhydrate
- 2-(1H-imidazol-5-yl)ethanamine dihydrochloride
- Histamine (dihydrochloride)
- NSC-257873
- 1H-Imidazole-5-ethanamine, hydrochloride (1:2)
- 2-(1H-imidazol-5-yl)ethan-1-amine dihydrochloride
- Histamine dihydrochloride (USAN)
- HISTAMINE DIHYDROCHLORIDE (USP-RS)
- HISTAMINE DIHYDROCHLORIDE [USP-RS]
- NSC257873
- HISTAMINE DIHYDROCHLORIDE (EP MONOGRAPH)
- HISTAMINE DIHYDROCHLORIDE [EP MONOGRAPH]
- SR-01000075270
- Bichlorhydrate d'histamine [French]
- Bichlorhydrate D'histamine
- Maxamine
- histamine dihcl
- Histamine6X
- Histamine dihydrochloride; 2-(1H-imidazol-4-yl)ethanamine dihydrochloride
- Histamine Phenolic
- Histamine1522
- Ceplene (TN)
- MFCD08060928
- MUSCLESHOK Sport
- Histamine dihydrocloride
- Histaminum hydrocloricum
- Dr. Freds Miracle Rub
- Bichlorhydrated'Histamine
- MUSCLESHOK Back Pain
- Pain Relieving Arthritis
- Australian Dream Back Pain
- CVS Arthritis Pain Relief
- Hand and Wrist Pain Cream
- ROPANA Pain Relief Cream
- 2-(1H-imidazol-5-yl)ethanamine;dihydrochloride
- C5H9N3.2HCl
- Histaminum Hydrochloricum 6C
- SCHEMBL58710
- SPECTRUM1500331
- Histaminum 30C Allergy Relief
- Histaminum Hydrochloricum200ck
- CHEMBL1533310
- DTXSID1058767
- SCHEMBL16778301
- HY-B0722R
- TIMTEC-BB SBB003722
- Histamine Dihydrochloride .025%
- Bakers Best Arthritis Pain Relief
- HMS1920D11
- Glucosamine Cream EXTRA STRENGTH
- BCP16313
- HY-B0722
- STR02021
- Tox21_500595
- CCG-38567
- HB2803
- s4118
- HISTAMINE DIHYDROCHLORIDE [MI]
- Walgreens Arthritis Pain Relief Cream
- AKOS015894236
- AKOS016353223
- AMINO ACTIVE Topical Analgesic Cream
- 1ST9098
- AC-7507
- BS-3141
- CS-W020699
- HAVASU HEMP Soothing Pain Relief Rub
- Histamine (dihydrochloride) (Standard)
- HISTAMINE HYDROCHLORIDE [MART.]
- KS-5310
- LP00595
- HISTAMINE HYDROCHLORIDE [WHO-DD]
- HISTAMINUM HYDROCHLORICUM [HPUS]
- NCGC00093973-01
- NCGC00093973-02
- NCGC00093973-03
- NCGC00261280-01
- 2-(4-Imidazolyl)ethylaminedihydrochloride
- 4-(2-aminoethyl)imidazole dihydorchloride
- BP-12001
- SY012860
- Mancore Muscle Mend Roll-ON Pain Reliever
- DB-019204
- HISTAMINE DIHYDROCHLORIDE [EMA EPAR]
- Histamine dihydrochloride, >=99.0% (AT)
- EU-0100595
- H0146
- NS00078856
- SW219552-1
- EN300-75558
- 4-(2-Aminoethyl)-1H-imidazole dihydrochloride
- D04444
- EDIHYDROCHLORIDEAVAILABLEFROM1KGTO100KG.
- H 7250
- H-2250
- H-2255
- 2-(1-H-imidazol-4-yl) Ethylamine Dihydrochloride
- Histamine dihydrochloride, >=99% (TLC), powder
- 1H-IMIDAZOLE-4-ETHANAMINE, CHLORIDE (1:1)
- Q5772985
- SR-01000075270-1
- SR-01000075270-7
- Histamine dihydrochloride, tested according to Ph Eur
- F2191-0277
- Histamine dihydrochloride, SAJ special grade, >=98.0%
- Histamine dihydrochloride, Vetec(TM) reagent grade, 98%
- Z1267773750
- 2-(1H-Imidazol-5-yl)ethanamine dihydrochloride, AldrichCPR
- 2-(1H-IMIDAZOL-5-YL)ETHANAMINE HYDROCHLORIDE (1:1)
- BAKERS BEST Arthritis Pain Relief Cream with Hemp and Lavender
- Histamine dihydrochloride 100 microg/mL in Acetonitrile/Water
- Histamine dihydrochloride, European Pharmacopoeia (EP) Reference Standard
- ALLERGEN SCRATCH EXTRACT POSITIVE CONTROL'TORII' HISTAMINE DIHYDROCHLORIDE
- Histamine dihydrochloride, United States Pharmacopeia (USP) Reference Standard
110.071275 100
154.990080 78.78
95.060342 51.86
199.967201 41.27
83.060349 41.07
201.982936 100
199.967219 68.48
110.071266 38.32
152.974523 16.79
62.018675 16.59
Histamine (has active moiety)
- Histamine dihydrochloride; menthol (component of)
- Capsaicin; histamine dihydrochloride; menthol (component of)
- Histamine Dihydrochloride; Lidocaine (component of)
- CAPSAICIN; histamine dihydrochloride (component of)
- Histamine dihydrochloride; menthol; methyl salicylate (component of)
- histamine dihydrochloride; menthol, unspecified form (component of)
- Acetylcholine chloride; histamine dihydrochloride; serotonin hydrochloride (component of)
- Galphimia glauca flowering top; histamine dihydrochloride; luffa operculata fruit; sulfur (component of)
- Dopamine; histamine dihydrochloride; phenethylamine hydrochloride; pork kidney; theanine (component of)
- Copper; estrone; histamine dihydrochloride; magnesium phosphate, tribasic, pentahydrate; sodium sulfate (component of)
- Cucumber; galphimia glauca flowering top; histamine dihydrochloride; onion; sodium chloride; strychnos nux-vomica seed (component of)
- Ambrosia artemisiifolia; apis mellifera; euphrasia stricta; histamine dihydrochloride; onion; schoenocaulon officinale seed; sulfur; valerian (component of)
- Anemone pulsatilla; arsenic trioxide; calcium sulfide; euphorbia resinifera resin; histamine dihydrochloride; iodine; lycopodium clavatum spore (component of)
- Ambrosia artemisiifolia; euphrasia stricta; histamine dihydrochloride; onion; schoenocaulon officinale seed; solidago virgaurea flowering top (component of)
- Eriodictyon californicum leaf; histamine dihydrochloride; ipecac; lobelia inflata; sambucus nigra flowering top; solidago virgaurea flowering top (component of)
- gamma-AMINOBUTYRIC ACID; ACETYLCHOLINE CHLORIDE; EPINEPHRINE; GOLDENSEAL; HISTAMINE DIHYDROCHLORIDE; NOREPINEPHRINE BITARTRATE; TARAXACUM OFFICINALE; TYRAMINE (component of)
- Calcium sulfide; euphorbia resinifera resin; histamine dihydrochloride; luffa operculata fruit; onion; pulsatilla vulgaris; schoenocaulon officinale seed (component of)
- Calcium fluoride; calcium phosphate; epinephrine; euphrasia officinalis leaf; histamine dihydrochloride; potassium chloride; silicon dioxide; sodium chloride (component of)
- Apis mellifera; euphrasia stricta; histamine dihydrochloride; naphthalene; onion; potassium iodide; schoenocaulon officinale seed; wyethia helenioides root (component of)
- Euphrasia stricta; histamine dihydrochloride; naphthalene; onion; potassium iodide; schoenocaulon officinale seed; urtica urens whole; wyethia helenioides root (component of)
- Arabica coffee bean; eriodictyon californicum leaf; histamine dihydrochloride; ipecac; lobelia inflata; matricaria chamomilla; sambucus nigra flowering top; solidago virgaurea flowering top (component of)
- Apis mellifera; apis mellifera venom; euphorbia resinifera resin; euphrasia stricta; hedera helix flowering twig; histamine dihydrochloride; onion; pulsatilla vulgaris; schoenocaulon officinale seed (component of)
- Bos taurus adrenal gland; corticotropin; histamine dihydrochloride; pork liver; rhus aromatica root bark; rhus glabra top; toxicodendron diversilobum leaf; toxicodendron pubescens leaf; urtica dioica whole (component of)
- Anemone pulsatilla; aralia racemosa root; euphrasia stricta; galphimia glauca flowering top; histamine dihydrochloride; luffa operculata fruit; onion; sodium chloride; solidago virgaurea flowering top (component of)
- Acetaldehyde; alcaligenes faecalis; candida albicans; candida parapsilosis; corticotropin human; coumarin; histamine dihydrochloride; lactic acid, L-; quercetin; saccharomyces cerevisiae; sepia officinalis juice (component of)
- Anemone pulsatilla; aralia racemosa root; calcium sulfide; euphorbia resinifera resin; galphimia glauca flowering top; goldenseal; histamine dihydrochloride; luffa operculata fruit; potassium dichromate; potassium iodide; teucrium marum (component of)
- Activated charcoal; amanita muscaria fruiting body; arabica coffee bean; avena sativa flowering top; bos taurus adrenal gland; corn silk; elymus repens whole; garlic; histamine dihydrochloride; hydrangea arborescens root; onion; skim milk (component of)
- gamma-AMINOBUTYRIC ACID; 5-METHOXYTRYPTAMINE; ACETYLCHOLINE CHLORIDE; DOPAMINE HYDROCHLORIDE; HISTAMINE DIHYDROCHLORIDE; LEVODOPA; MELATONIN; NOREPINEPHRINE BITARTRATE; PHENETHYLAMINE HYDROCHLORIDE; SEROTONIN HYDROCHLORIDE; TAURINE; TYRAMINE (component of)
- Aloe; arsenic trioxide; conium maculatum flowering top; euphorbia resinifera resin; formic acid; galium aparine; histamine dihydrochloride; phytolacca americana root; taraxacum officinale; thuja occidentalis leafy twig; viscum album fruiting top; zinc (component of)
- Allium cepa whole; ambrosia artemisiifolia; euphrasia stricta; fomitopsis pinicola fruiting body; galphimia glauca flowering top; histamine dihydrochloride; hydrastis canadensis whole; lemna minor; luffa operculata fruit; schoenocaulon officinale seed; sodium chloride (component of)
- Anthoxanthum odoratum pollen; bos taurus adrenal gland; corticotropin; cynodon dactylon whole; dactylis glomerata top; histamine dihydrochloride; lolium perenne top; paspalum notatum pollen; phleum pratense top; poa pratensis top; pork liver; sorghum halepense pollen (component of)
- Antimony trisulfide; arctium lappa root; arnica montana root; formic acid; graphite; histamine dihydrochloride; ledum palustre twig; lycopodium clavatum spore; pine tar; selenium; strychnos ignatii seed; sulfur; sulfuric acid; tellurium; thuja occidentalis leafy twig (component of)
- Alternaria alternata; aspergillus niger var. niger; bos taurus adrenal gland; candida albicans; candida parapsilosis; corticotropin; dermatophagoides pteronyssinus; histamine dihydrochloride; house dust; mucor racemosus; pork liver; rhizopus stolonifer; saccharomyces cerevisiae (component of)
- Ambrosia artemisiifolia whole; apis mellifera; bos taurus adrenal gland; echinacea angustifolia whole; ephedra distachya flowering twig; epinephrine; euphrasia stricta; galium aparine whole; goldenseal; histamine dihydrochloride; onion; pork liver; schoenocaulon officinale seed (component of)
- Alternaria alternata; aspergillus niger var. niger; beef liver; bos taurus adrenal gland; candida albicans; candida parapsilosis; corticotropin; dermatophagoides pteronyssinus; histamine dihydrochloride; house dust; mucor racemosus; rhizopus stolonifer; saccharomyces cerevisiae (component of)
- Antimony trisulfide; arctium lappa root; arnica montana root; euphrasia stricta; formic acid; graphite; histamine dihydrochloride; ledum palustre twig; lycopodium clavatum spore; pine tar; selenium; strychnos ignatii seed; sulfur; sulfuric acid; tellurium; thuja occidentalis leafy twig (component of)
- Ambrosia artemisiifolia whole; arsenic trioxide; baptisia tinctoria root; echinacea angustifolia whole; euphrasia stricta; histamine dihydrochloride; onion; phosphorus; pulsatilla pratensis whole; sodium chloride; sodium sulfate; solidago virgaurea flowering top; strychnos nux-vomica seed; sulfur (component of)
- Alternaria alternata; aspergillus fumigatus; aspergillus niger var. niger; aureobasidium pullulans var. pullutans; boletus satanas fruiting body; botrytis cinerea; candida albicans; cochliobolus sativus; fusarium oxysporum; histamine dihydrochloride; passalora fulva; rhizopus stolonifer; ustilago maydis (component of)
- Agrimonia eupatoria; anemone pulsatilla; bellis perennis; bos taurus adrenal gland; camphor (natural); canis lupus familiaris milk; equisetum hyemale; formica rufa; fraxinus americana bark; histamine dihydrochloride; iodine; matricaria chamomilla; potassium dichromate; salix nigra bark; sodium chloride; tobacco leaf (component of)
- Antimony trisulfide; apis mellifera; capsicum; fucus vesiculosus; galium aparine; garcinia gummi-gutta fruit; ginger; hamamelis virginiana root bark/stem bark; histamine dihydrochloride; petroselinum crispum; pork liver; strychnos nux-vomica seed; sus scrofa pancreas; thuja occidentalis leafy twig; thyroid, porcine (component of)
- Antipyrine; apis mellifera; calendula officinalis flowering top; copaifera officinalis resin; croton tiglium seed; daphne mezereum bark; delphinium staphisagria seed; graphite; histamine dihydrochloride; kerosene; lytta vesicatoria; rhododendron tomentosum leafy twig; sodium chloride; sulfur; toxicodendron vernix leafy twig (component of)
- Ambrosia artemisiifolia whole; arisaema triphyllum root; arsenic trioxide; arundo pliniana root; euphrasia stricta; galphimia glauca flowering top; histamine dihydrochloride; lobaria pulmonaria; naphthalene; onion; potassium dichromate; schoenocaulon officinale seed; sodium chloride; solidago virgaurea flowering top; wyethia helenioides root (component of)
- Calcium sulfide; cupressus sempervirens fruiting leafy twig; eucalyptus globulus leaf; euphorbia resinifera resin; galphimia glauca flowering top; goldenseal; histamine dihydrochloride; luffa operculata fruit; mentha piperita; origanum majorana whole; pinus sylvestris leafy twig; potassium dichromate; potassium iodide; pulsatilla vulgaris; sage (component of)
- Anas platyrhynchos feather; anser anser feather; bos taurus adrenal gland; bos taurus hair; canis lupus familiaris hair; cavia porcellus hair; corticotropin; equus caballus dander; felis catus hair; gallus gallus feather; histamine dihydrochloride; oryctolagus cuniculus hair; pork liver; rattus norvegicus hair; serinus canaria feather; sus scrofa hair (component of)
- Ambrosia artemisiifolia whole; anthoxanthum odoratum; arsenic trioxide; beef liver; cynodon dactylon pollen; dactylis glomerata top; epinephrine; histamine dihydrochloride; lolium perenne pollen; paspalum notatum pollen; phleum pratense top; phosphorus; poa pratensis top; potassium gluconate; pulsatilla pratensis whole; sorghum halepense pollen; sulfur (component of)
- Ambrosia artemisiifolia; anguilla rostrata blood serum; arundo pliniana root; asclepias curassavica; black currant; citric acid monohydrate; histamine dihydrochloride; human interleukin 12; interferon gamma-1B; manganese gluconate; parietaria officinalis; phleum pratense; sodium pyruvate; sodium sulfate; succinic acid; sulfur; urtica urens; viburnum opulus root; wyethia helenioides root (component of)
- Apis mellifera; arnica montana; bacillus anthracis immunoserum rabbit; calendula officinalis flowering top; citharacanthus spinicrus; conium maculatum flowering top; echinacea angustifolia; euphorbia hirta flowering top; grindelia hirsutula flowering top; histamine dihydrochloride; hypericum perforatum; lachesis muta venom; latrodectus mactans; ledum palustre twig; rancid beef (component of)
- Aconitum napellus; american ginseng; arsenic trioxide; baptisia tinctoria root; bryonia alba root; cinchona officinalis bark; echinacea, unspecified; eupatorium perfoliatum flowering top; gelsemium sempervirens root; histamine dihydrochloride; influenza A virus; influenza B virus; ipecac; phosphorus; potassium carbonate; strychnos nux-vomica seed; toxicodendron pubescens leaf (component of)
- gamma-AMINOBUTYRIC ACID; APIOLE (PARSLEY); ASPARAGINE; BENZOIC ACID; CHOLINE HYDROXIDE; CINNAMIC ACID; COUMARIN; ESTRONE; EUGENOL; FOLIC ACID; FORMALDEHYDE; GOLDENSEAL; HISTAMINE DIHYDROCHLORIDE; LACTOSE, UNSPECIFIED FORM; MENADIONE; PHENYL ISOTHIOCYANATE; PIPERINE; PROGESTERONE; QUERCETIN; RUTIN; SUS SCROFA OVARY; TARAXACUM OFFICINALE; TYRAMINE; VANILLYLAMINE; XANTHINE (component of)
- Ambrosia artemisiifolia whole; arsenic trioxide; baptisia tinctoria root; black mustard seed; echinacea angustifolia whole; epinephrine; euphrasia stricta; histamine dihydrochloride; onion; phosphorus; pulsatilla pratensis whole; schoenocaulon officinale seed; sodium chloride; sodium sulfate; solidago virgaurea flowering top; strychnos nux-vomica seed; sulfur; wyethia helenioides root (component of)
- Anhydrous citric acid; arsenic trioxide; benzoic acid; berberis vulgaris root bark; betula pendula leaf; chelidonium majus; chlorine; garlic; histamine dihydrochloride; ichthammol; juniperus communis stem; lycopodium clavatum spore; mercurius solubilis; oyster shell calcium carbonate, crude; phenol; sodium bicarbonate; sodium hypochlorite; solidago virgaurea flowering top; sulfur (component of)
- Calcium iodide; calcium sulfate anhydrous; calcium sulfide; echinacea angustifolia; echinacea purpurea; euphorbia resinifera resin; goldenseal; histamine dihydrochloride; lactose, unspecified form; luffa operculata fruit; mercuric sulfide; onion; phosphorus; potassium chloride; potassium dichromate; potassium sulfate; pulsatilla vulgaris; skim milk; thuja occidentalis leafy twig (component of)
- Acetaldehyde; acetylcholine chloride; caffeic acid; candida albicans; chlorogenic acid; cinnamic acid; coumarin; cysteine; dopamine hydrochloride; epinephrine; gallic acid monohydrate; histamine dihydrochloride; indole; malvin; mannose, D-; menadione; norepinephrine bitartrate; phenyl isothiocyanate; phlorizin; pork liver; quercetin; serotonin hydrochloride; sus scrofa pancreas; tyramine (component of)
- Ambrosia artemisiifolia; anemone pulsatilla; apis mellifera; arsenic triiodide; arsenic trioxide; echinacea angustifolia; euphrasia stricta; garlic; histamine dihydrochloride; iodine; lycopodium clavatum spore; mercurius solubilis; onion; schoenocaulon officinale seed; solidago virgaurea flowering top; strychnos nux-vomica seed; sulfur; sus scrofa pituitary gland, posterior; toxicodendron pubescens leaf (component of)
- AMYL NITRITE; ARSENIC TRIOXIDE; CANIS LUPUS FAMILIARIS MILK; DERMATOPHAGOIDES FARINAE; EUPHRASIA STRICTA; FELIS CATUS MILK; HISTAMINE DIHYDROCHLORIDE; HYALURONIDASE; INTERFERON gamma PORCINE RECOMBINANT; IRIDIUM; ONION; PERIPLANETA AMERICANA; POTASSIUM CHLORIDE; SCHOENOCAULON OFFICINALE SEED; SELENIUM; SEROTONIN HYDROCHLORIDE; SUS SCROFA ADRENAL GLAND; SUS SCROFA NASAL MUCOSA; WYETHIA HELENIOIDES ROOT (component of)
- Alternaria alternata; aspergillus fumigatus; aspergillus niger var. niger; aureobasidium pullulans var. pullutans; boletus satanas fruiting body; botrytis cinerea; candida albicans; cochliobolus sativus; fusarium oxysporum; gelsemium sempervirens root; histamine dihydrochloride; passalora fulva; potassium chloride; potassium dichromate; rhizopus stolonifer; schoenocaulon officinale seed; ustilago maydis (component of)
- Acer saccharum pollen; alnus rubra pollen; betula pendula pollen; bos taurus adrenal gland; carya glabra pollen; corticotropin; corylus avellana pollen; fagus sylvatica pollen; fraxinus excelsior pollen; histamine dihydrochloride; juniperus virginiana pollen; platanus X acerifolia pollen; populus alba pollen; pork liver; quercus robur pollen; salix X fragilis pollen; sambucus canadensis flower; ulmus glabra pollen (component of)
- Alfalfa; ambrosia artemisiifolia; anhydrous citric acid; bryonia alba root; coumarin; euphrasia stricta; goldenseal; histamine dihydrochloride; juniper berry; onion; phosphorus; pork liver; pulsatilla vulgaris; sodium sulfate; solidago virgaurea flowering top; sulfur; sus scrofa adrenal gland; sus scrofa nasal mucosa; symplocarpus foetidus root; tanacetum vulgare top; taraxacum officinale; trifolium pratense flower (component of)
- American ginseng; arsenic trioxide; bryonia alba root; calcium sulfide; echinacea, unspecified; euphrasia stricta; gaultheria procumbens top; gelsemium sempervirens root; goldenseal; histamine dihydrochloride; influenza A virus; influenza B virus; lobaria pulmonaria; mercury; phosphorus; potassium dichromate; pulsatilla vulgaris; sage; schoenocaulon officinale seed; sinusitisinum; toxicodendron pubescens leaf; verbascum thapsus (component of)
- Balsam peru; bryonia alba root; calcium acetate; calomel; echinacea angustifolia; echinacea purpurea; eucalyptus globulus leaf; euphorbia resinifera resin; euphrasia stricta; histamine dihydrochloride; ipecac; luffa operculata fruit; mercuric cyanide; mercuric sulfide; pancrelipase; potassium dichromate; protortonia cacti; sambucus nigra flowering top; sanguinarine nitrate; saponaria officinalis root; sodium arsenate, dibasic, heptahydrate; sodium borate (component of)
- Adenosine cyclic phosphate; arisaema triphyllum root; arundo pliniana root; collinsonia canadensis root; corticotropin; cortisone acetate; epinephrine; euphrasia stricta; galphimia glauca flowering top; histamine dihydrochloride; hyacinthoides non-scripta whole; onion; pervinca minor whole; potassium chloride; rumex crispus root; saccharomyces cerevisiae rna; sage; schoenocaulon officinale seed; sodium chloride; tanacetum vulgare top; trifolium pratense flower (component of)
- Aloe; berberis vulgaris root bark; betula pendula leaf; bryonia alba root; echinacea angustifolia; frangula purshiana bark; ginkgo; histamine dihydrochloride; juniperus communis stem; lycopodium clavatum spore; milk thistle; quercus robur flower bud; ribes nigrum flower bud; rosa canina leaf; rosmarinus officinalis flowering top; scrophularia nodosa; senna leaf; solidago virgaurea flowering top; taraxacum officinale; tea tree oil; vaccinium vitis-idaea leaf (component of)
- Anemone pulsatilla; arsenic trioxide; atropa belladonna; bryonia alba root; calcium sulfide; camphor (natural); canis lupus familiaris milk; drosera anglica; echinacea, unspecified; goldenseal; helianthemum canadense; histamine dihydrochloride; iodine; lobaria pulmonaria; phosphorus; potassium dichromate; potassium iodide; schoenocaulon officinale seed; silicon dioxide; strychnos ignatii seed; strychnos nux-vomica seed; sulfur; teucrium marum; verbascum thapsus (component of)
- Aconitum napellus whole; apis mellifera; avena sativa flowering top; clematis vitalba flower; dibasic potassium phosphate; helianthemum canadense; histamine dihydrochloride; impatiens glandulifera flower; lilium lancifolium whole flowering; matricaria chamomilla whole; medicago sativa whole; ornithogalum umbellatum flowering top; passiflora incarnata flowering top; phosphorus; prunus cerasifera flower; pulsatilla vulgaris whole; strychnos ignatii seed; valerian (component of)
- Aconitum napellus; american ginseng; anemone pulsatilla; arsenic trioxide; bryonia alba root; echinacea, unspecified; euphrasia stricta; gaultheria procumbens top; gelsemium sempervirens root; goldenseal; histamine dihydrochloride; influenza A virus; influenza B virus; lobaria pulmonaria; lycopodium clavatum spore; phosphorus; potassium dichromate; sage; sinusitisinum; strychnos nux-vomica seed; thuja occidentalis leafy twig; toxicodendron pubescens leaf; verbascum thapsus (component of)
- gamma-AMINOBUTYRIC ACID; ACETYLCHOLINE CHLORIDE; ADENOSINE TRIPHOSPHATE DISODIUM; AETHUSA CYNAPIUM WHOLE; ALUMINUM OXIDE; ARSENIC TRIOXIDE; CORTISONE ACETATE; DIBASIC POTASSIUM PHOSPHATE; DOPAMINE HYDROCHLORIDE; GINKGO; HISTAMINE DIHYDROCHLORIDE; LEVOTHYROXINE; LYCOPODIUM CLAVATUM SPORE; MELATONIN; NADIDE; NOREPINEPHRINE BITARTRATE; OXYTOCIN; PHOSPHORUS; SEMECARPUS ANACARDIUM JUICE; SEROTONIN HYDROCHLORIDE; SUS SCROFA CEREBRUM; SUS SCROFA HYPOTHALAMUS; UBIDECARENONE; ZINC (component of)
- Adenosine cyclic phosphate; ageratina aromatica root; arisaema triphyllum root; arundo pliniana root; asarum canadense root; corticotropin; cortisone acetate; epinephrine; euphrasia stricta; fraxinus americana bark; galphimia glauca flowering top; helianthus annuus flowering top; histamine dihydrochloride; jacobaea vulgaris whole; juniperus virginiana oil; onion; plantago major whole; potassium chloride; rumex crispus root; saccharomyces cerevisiae rna; schoenocaulon officinale seed; sodium chloride (component of)
- Adenosine cyclic phosphate; ageratina aromatica root; arisaema triphyllum root; arundo pliniana root; asarum canadense root; corticotropin; cortisone acetate; epinephrine; euphrasia stricta; fraxinus americana bark; galphimia glauca flowering top; helianthus annuus flowering top; histamine dihydrochloride; jacobaea vulgaris whole; juniperus virginiana twig; onion; plantago major whole; potassium chloride; rumex crispus root; saccharomyces cerevisiae rna; schoenocaulon officinale seed; sodium chloride (component of)
- Adenosine cyclic phosphate; ambrosia artemisiifolia whole; arisaema triphyllum root; arundo pliniana root; corticotropin; cortisone acetate; datura stramonium; elymus repens root; epinephrine; erechtites hieraciifolius whole; erigeron canadensis whole; euonymus atropurpureus branch bark/root bark; euphrasia stricta; galphimia glauca flowering top; histamine dihydrochloride; medicago sativa whole; onion; potassium chloride; saccharomyces cerevisiae rna; schoenocaulon officinale seed; sodium chloride (component of)
- Adenosine cyclic phosphate; ambrosia artemisiifolia whole; arisaema triphyllum root; arundo pliniana root; caulophyllum thalictroides root; claviceps purpurea sclerotium; conium maculatum flowering top; corticotropin; cortisone acetate; epinephrine; euphrasia stricta; fagopyrum esculentum whole; galphimia glauca flowering top; histamine dihydrochloride; melilotus officinalis top; onion; potassium chloride; rumex crispus root; saccharomyces cerevisiae rna; schoenocaulon officinale seed; sodium chloride; wormwood (component of)
- Adenosine cyclic phosphate; alnus serrulata bark; arisaema triphyllum root; arundo pliniana root; centaurea benedicta; chicory root; corticotropin; cortisone acetate; epinephrine; euphrasia stricta; galphimia glauca flowering top; histamine dihydrochloride; juglans cinerea branch bark/root bark; mucuna pruriens fruit trichome; nasturtium officinale; onion; potassium chloride; saccharomyces cerevisiae rna; sassafras albidum root bark; schoenocaulon officinale seed; sodium chloride; toxicodendron pubescens leaf (component of)
- Aconitum napellus; apis mellifera; arnica montana; arsenic trioxide; atropa belladonna; bellis perennis; bryonia alba root; calendula officinalis flowering top; daphne mezereum bark; ferrosoferric phosphate; helianthemum canadense; histamine dihydrochloride; hypericum perforatum; lactic acid, L-; matricaria chamomilla; ornithogalum umbellatum; passiflora incarnata flowering top; phosphorus; prunus spinosa flower bud; strychnos ignatii seed; sulfur; toxicodendron pubescens leaf; valerian; veratrum album root (component of)
- Anemone pulsatilla; antimony trisulfide; apis mellifera; arsenic trioxide; berberis vulgaris root bark; bryonia alba root; chelidonium majus; croton tiglium seed; daphne mezereum bark; echinacea, unspecified; graphite; histamine dihydrochloride; kerosene; lycoperdon utriforme fruiting body; lycopodium clavatum spore; lytta vesicatoria; pulex irritans; rumex crispus root; smilax ornata root; solanum dulcamara top; strychnos nux-vomica seed; sulfur; taraxacum officinale; toxicodendron pubescens leaf; urtica urens (component of)
- Achillea millefolium whole; adenosine cyclic phosphate; arisaema triphyllum root; arundo pliniana root; bellis perennis whole; conium maculatum flowering top; corticotropin; cortisone acetate; epinephrine; equisetum hyemale whole; euphrasia stricta; fagopyrum esculentum whole; galphimia glauca flowering top; histamine dihydrochloride; medicago sativa whole; onion; potassium chloride; prunus spinosa flower bud; saccharomyces cerevisiae rna; schoenocaulon officinale seed; sodium chloride; solidago virgaurea flowering top (component of)
- Anemone pulsatilla; antimony potassium tartrate; arctium lappa root; arsenic trioxide; bryonia alba root; chelidonium majus; cinchona officinalis bark; drosera anglica; echinacea, unspecified; eucalyptus gum; goldenseal; histamine dihydrochloride; influenza A virus; influenza B virus; myrrh; phosphorus; potassium carbonate; potassium dichromate; potassium iodide; rumex crispus root; sambucus nigra flowering top; sodium sulfate; spongia officinalis skeleton, roasted; strychnos nux-vomica seed; tussilago farfara; verbascum thapsus (component of)
- Activated charcoal; alfalfa; american ginseng; asparagus; avena sativa flowering top; beef kidney; beef liver; berberis vulgaris root bark; bos taurus adrenal gland; bos taurus colon; bos taurus lymph vessel; bos taurus pancreas; carpinus betulus flower; garlic; gentiana lutea root; histamine dihydrochloride; juniperus communis stem; lactic acid, DL-; malus sylvestris flower; passiflora incarnata flowering top; phosphorus; ribes nigrum flower bud; scrophularia nodosa; solidago virgaurea flowering top; tribasic calcium phosphate (component of)
- Adenosine cyclic phosphate; ambrosia artemisiifolia whole; arisaema triphyllum root; arundo pliniana root; chelidonium majus whole; chenopodium vulvaria whole; chicory root; corticotropin; cortisone acetate; epinephrine; euphrasia stricta; galphimia glauca flowering top; hedera helix flowering twig; histamine dihydrochloride; juglans cinerea branch bark/root bark; onion; potassium chloride; pulsatilla vulgaris whole; saccharomyces cerevisiae rna; schoenocaulon officinale seed; sodium chloride; teucrium scorodonia flowering top (component of)
- Aconitum napellus; apis mellifera; arnica montana; arsenic trioxide; atropa belladonna; bellis perennis; bryonia alba root; calendula officinalis flowering top; clematis recta flowering top; comfrey root; ferrosoferric phosphate; helianthemum canadense; histamine dihydrochloride; hypericum perforatum; impatiens glandulifera flower; matricaria chamomilla; ornithogalum umbellatum; passiflora incarnata flowering top; phosphorus; prunus cerasifera flower; strychnos ignatii seed; sulfur; toxicodendron pubescens leaf; veratrum album root (component of)
- Adenosine cyclic phosphate; agrimonia eupatoria whole; agrostemma githago seed; ailanthus altissima flowering twig; arisaema triphyllum root; arundo pliniana root; corticotropin; cortisone acetate; cynodon dactylon whole; epinephrine; equisetum hyemale whole; euphrasia stricta; galphimia glauca flowering top; histamine dihydrochloride; onion; platanus occidentalis whole; populus balsamifera leaf bud; potassium chloride; saccharomyces cerevisiae rna; schoenocaulon officinale seed; sodium chloride; solidago virgaurea flowering top (component of)
- Aconitum napellus; arsenic trioxide; bryonia alba root; calcium fluoride; ceanothus americanus leaf; centella asiatica; cinchona officinalis bark; convallaria majalis; digitalis; echinacea, unspecified; gelsemium sempervirens root; histamine dihydrochloride; lachesis muta venom; naja naja venom; nitroglycerin; phosphorus; potassium carbonate; pulsatilla vulgaris; selenicereus grandiflorus stem; sodium carbonate; sodium chloride; strophanthus hispidus seed; strychnos nux-vomica seed; sulfur; thyroid, unspecified; toxicodendron pubescens leaf (component of)
- Antimony trisulfide; apis mellifera; arsenic trioxide; baptisia tinctoria root; blatta orientalis; bromine; bryonia alba root; candida albicans; canis lupus familiaris hair; centaurea benedicta; chelidonium majus; cinchona officinalis bark; claviceps purpurea sclerotium; felis catus hair; histamine dihydrochloride; house dust; ipecac; iris versicolor root; lycopodium clavatum spore; podophyllum; saccharomyces cerevisiae; silicon dioxide; solidago virgaurea flowering top; strychnos nux-vomica seed; toxicodendron pubescens leaf; ustilago maydis (component of)
- Acetaldehyde; acetic acid; alpine strawberry; anhydrous citric acid; caffeine; calcium sulfate anhydrous; cortisone acetate; cow milk; echinacea purpurea; egg phospholipids; estrone; fumaric acid; gallic acid monohydrate; histamine dihydrochloride; hydrangea arborescens root; limulus polyphemus; lycopodium clavatum spore; paraffin; phosphoric acid; pork liver; saccharomyces cerevisiae; silicon dioxide; silver nitrate; sodium bicarbonate; sodium chloride; solanum lycopersicum; strychnos nux-vomica seed; sucrose; sus scrofa adrenal gland; tartaric acid (component of)
- Adenosine triphosphate disodium; anthraquinone; ascorbic acid; aspirin; bilberry; colchicum autumnale bulb; conium maculatum flowering top; galium aparine; goldenseal; histamine dihydrochloride; hydroquinone; lactic acid, L-; magnesium gluconate; manganese phosphate, dibasic; nadide; naphthoquine; niacinamide; norepinephrine bitartrate; pantothenic acid; podophyllum; pyridoxine hydrochloride; riboflavin; salmonella enterica subsp. enterica serovar enteritidis; sodium diethyl oxalacetate; sulfur; thiamine hydrochloride; thioctic acid; ubidecarenone (component of)
- Apis mellifera; capsicum; chaste tree fruit; chelidonium majus whole; colchicum autumnale bulb; dibasic potassium phosphate; fucus vesiculosus; galium aparine whole; graphite; histamine dihydrochloride; lycopodium clavatum spore; magnesium phosphate, dibasic trihydrate; oyster shell calcium carbonate, crude; phytolacca americana root; pulsatilla pratensis whole; semecarpus anacardium juice; smilax ornata root; sodium phosphate, dibasic, heptahydrate; strychnos nux-vomica seed; taraxacum officinale; ubidecarenone; urtica dioica whole; zanthoxylum americanum bark (component of)
- 1,4-Naphthoquinone; adenosine triphosphate disodium; ascorbic acid; aspirin; bacillus anthracis immunoserum rabbit; bilberry; colchicum autumnale bulb; conium maculatum flowering top; galium aparine; goldenseal; histamine dihydrochloride; hydroquinine; lactic acid, L-; magnesium gluconate; manganese phosphate, dibasic; nadide; niacinamide; norepinephrine; pantothenic acid; podophyllum; pyridoxine hydrochloride; riboflavin; salmonella enterica subsp. enterica serovar enteritidis; sodium diethyl oxalacetate; sulfur; thiamine hydrochloride; thioctic acid; ubidecarenone (component of)
- gamma-AMINOBUTYRIC ACID; ACETALDEHYDE; ACETYLCHOLINE CHLORIDE; ASCORBIC ACID; ASPARTAME; BUTYLATED HYDROXYTOLUENE; CAFFEIC ACID; CANDIDA ALBICANS; CHLOROGENIC ACID; CINNAMIC ACID; CONIFERYL ALCOHOL; COUMARIN; DOPAMINE HYDROCHLORIDE; ESTRADIOL; GALLIC ACID MONOHYDRATE; HISTAMINE DIHYDROCHLORIDE; HYDROCORTISONE; INDOLE; LEVODOPA; MALVIN; MANNOSE, D-; MELATONIN; MENADIONE; NOREPINEPHRINE BITARTRATE; OXITRIPTAN; PETROSELINUM CRISPUM; PHENYL ISOTHIOCYANATE; PHENYLALANINE; PHLORIZIN; PIPERINE; PROGESTERONE; PYRROLE; QUERCETIN; RUTIN; SEROTONIN HYDROCHLORIDE; TAURINE; TYRAMINE (component of)
- gamma-AMINOBUTYRIC ACID; ACETALDEHYDE; ACETYLCHOLINE CHLORIDE; APIOLE (PARSLEY); ASCORBIC ACID; ASPARTAME; BUTYLATED HYDROXYTOLUENE; CAFFEIC ACID; CANDIDA ALBICANS; CHLOROGENIC ACID; CINNAMIC ACID; CONIFERYL ALCOHOL; COUMARIN; DOPAMINE HYDROCHLORIDE; ESTRADIOL; GALLIC ACID MONOHYDRATE; HISTAMINE DIHYDROCHLORIDE; HYDROCORTISONE; INDOLE; LEVODOPA; MALVIN; MELATONIN; MENADIONE; NOREPINEPHRINE BITARTRATE; OCTOPAMINE HYDROCHLORIDE; OXITRIPTAN; PHENYL ISOTHIOCYANATE; PHENYLALANINE; PHLORIZIN; PIPERINE; PROGESTERONE; PYRROLE; QUERCETIN; RUTIN; SALSOLINOL HYDROCHLORIDE; SEROTONIN HYDROCHLORIDE; TAURINE; YEAST MANNAN (component of)
- Aconitum napellus whole; apis mellifera; arnica montana whole; arsenic trioxide; atropa belladonna; bellis perennis whole; bryonia alba root; calendula officinalis flowering top; clematis recta flowering top; clematis vitalba flower; comfrey root; ferrosoferric phosphate; helianthemum canadense; histamine dihydrochloride; hypericum perforatum; impatiens glandulifera flower; matricaria chamomilla whole; ornithogalum umbellatum flowering top; passiflora incarnata flowering top; phosphorus; prunus cerasifera flower; strychnos ignatii seed; sulfur; toxicodendron pubescens leaf; trifolium pratense flower; veratrum album root (component of)
- alpha-KETOGLUTARIC ACID; alpha-LIPOIC ACID; ACETALDEHYDE; AVENA SATIVA FLOWERING TOP; CHELIDONIUM MAJUS; CHOLESTEROL; CINCHONA OFFICINALIS BARK; CYNARA SCOLYMUS LEAF; FORMIC ACID; HISTAMINE DIHYDROCHLORIDE; LYCOPODIUM CLAVATUM SPORE; MALIC ACID; MENADIONE; METHYLCOBALAMIN; MILK THISTLE; OROTIC ACID MONOHYDRATE; OYSTER SHELL CALCIUM CARBONATE, CRUDE; PORK INTESTINE; PORK LIVER; PROTEUS MORGANII; SALMONELLA ENTERICA SUBSP. ENTERICA SEROVAR ENTERITIDIS; SODIUM DIETHYL OXALACETATE; SULFUR; SUS SCROFA COLON; SUS SCROFA DUODENUM; SUS SCROFA GALLBLADDER; SUS SCROFA LYMPH; SUS SCROFA PANCREAS; SUS SCROFA THYMUS; TARAXACUM OFFICINALE; VERATRUM ALBUM ROOT (component of)
- ALMOND; APPLE; ARSENIC TRIOXIDE; ATLANTIC COD; ATLANTIC SALMON; BANANA; BEEF; BEEF LIVER; BLACK PEPPER; BLACK WALNUT; BOS TAURUS ADRENAL GLAND; BRAZIL NUT; CANOLA OIL; CASEIN, LACTOCOCCUS LACTIS CULTURED, PENICILLIUM ROQUEFORTI CULTURED, AGED; CASHEW; CHICKEN; COMMON SHRIMP; CORTISONE ACETATE; COW MILK; EDIBLE ROCK CRAB; EGG; EGGPLANT; ENGLISH WALNUT; HISTAMINE DIHYDROCHLORIDE; HYALURONIDASE; INTERFERON gamma PORCINE RECOMBINANT; KIDNEY BEAN; LAMB; LYCOPODIUM CLAVATUM SPORE; NORTHERN BLUEFIN TUNA; ONION; ORANGE; PACIFIC OYSTER; PEANUT; PECAN; PHOSPHORUS; PORK; POTASSIUM GLUCONATE; POTATO; SEROTONIN HYDROCHLORIDE; STRAWBERRY; STRYCHNOS NUX-VOMICA SEED; TOMATO; WHEAT (component of)
- Achillea millefolium; aconitum napellus; apis mellifera; arnica montana; arsenic trioxide; atropa belladonna; bellis perennis; bryonia alba root; calendula officinalis flowering top; citrullus colocynthis fruit pulp; clematis vitalba flower; comfrey root; echinacea angustifolia; echinacea purpurea; eupatorium perfoliatum flowering top; ferrosoferric phosphate; hamamelis virginiana root bark/stem bark; helianthemum nummularium flower; histamine dihydrochloride; hypericum perforatum; impatiens glandulifera flower; matricaria chamomilla; ornithogalum umbellatum flowering top; prunus cerasifera flower; solanum dulcamara whole; sulfur; toxicodendron pubescens leaf; veratrum album root (component of)
- Antirrhinum majus leaf; beef liver; C12-17 alkane; calendula officinalis flowering top; camellia chekiangoleosa seed oil; corticotropin; delphinium ajacis seed; dianthus caryophyllus whole; dianthus superbus flowering top; eschscholzia californica flowering top; gardenia jasminoides fruit; gentiana lutea root; gladiolus communis whole; hedychium flavescens whole; histamine dihydrochloride; kalimeris indica whole; liriodendron tulipifera whole; lonicera canadensis whole; lupinus elegans whole; narcissus poeticus whole; nerium oleander whole; paeonia X suffruticosa seed; ruellia simplex whole; sedum roseum whole; sus scrofa adrenal gland; symphyotrichum oblongifolium whole; townsendia exscapa whole; viola tricolor whole (component of)
- gamma-AMINOBUTYRIC ACID; ACTIVATED CHARCOAL; ADENOSINE TRIPHOSPHATE DISODIUM; APIOLE (PARSLEY); ASPARAGINE MONOHYDRATE; BENZOIC ACID; CALCIUM CITRATE; CALCIUM FLUORIDE; CALCIUM GLUCONATE; CANIS LUPUS FAMILIARIS MILK; CHOLINE HYDROXIDE; CINNAMIC ACID; COUMARIN; COW MILK; ESTRONE; EUGENOL; FELIS CATUS MILK; FOLIC ACID; FORMALDEHYDE; HISTAMINE DIHYDROCHLORIDE; HUMAN MILK; LACTIC ACID, L-; LACTOSE, UNSPECIFIED FORM; MATRICARIA RECUTITA; MENADIONE; OYSTER SHELL CALCIUM CARBONATE, CRUDE; PHENYL ISOTHIOCYANATE; PIPERINE; QUERCETIN; RUTIN; SALMONELLA ENTERICA SUBSP. ENTERICA SEROVAR ENTERITIDIS; SKIM MILK; SUS SCROFA ILEUM; SUS SCROFA JEJUNUM; SUS SCROFA OVARY; SUS SCROFA STOMACH; TOXICODENDRON PUBESCENS LEAF; TRIBASIC CALCIUM PHOSPHATE (component of)
- APPLE; ARSENIC TRIOXIDE; ATLANTIC COD; ATLANTIC SALMON; BANANA; BEEF; BITTER ALMOND; BLACK WALNUT; BOS TAURUS ADRENAL GLAND; BRAZIL NUT; CANOLA OIL; CASEIN, LACTOCOCCUS LACTIS CULTURED, PENICILLIUM ROQUEFORTI CULTURED, AGED; CASHEW; CHICKEN; COCONUT; CORTISONE ACETATE; COW MILK; CRANGON SHRIMP; EDIBLE ROCK CRAB; EGG; EGGPLANT; ENGLISH WALNUT; FLAX SEED; FRAGARIA VESCA FRUIT; GREEN PEPPERCORN; HEPARIN, BOVINE; HISTAMINE DIHYDROCHLORIDE; HYALURONIDASE (BOVINE); INTERLEUKIN-1 beta HUMAN; KIDNEY BEAN; LAMB; LYCOPODIUM CLAVATUM SPORE; NORTHERN BLUEFIN TUNA; ONION; ORANGE; PACIFIC OYSTER; PEANUT; PECAN; PHOSPHORUS; PORK; POTASSIUM GLUCONATE; SEROTONIN HYDROCHLORIDE; SESAME SEED; SOLANUM TUBEROSUM WHOLE; STRYCHNOS NUX-VOMICA SEED; TOMATO; WHEAT (component of)
- gamma-AMINOBUTYRIC ACID; ACTIVATED CHARCOAL; ADENOSINE TRIPHOSPHATE DISODIUM; ASPARAGINE MONOHYDRATE; BENZOIC ACID; CALCIUM CITRATE; CALCIUM FLUORIDE; CALCIUM GLUCONATE; CANIS LUPUS FAMILIARIS MILK; CHOLINE HYDROXIDE; CINNAMIC ACID; COUMARIN; COW MILK; ESTRONE; EUGENOL; FELIS CATUS MILK; FOLIC ACID; FORMALDEHYDE; HISTAMINE DIHYDROCHLORIDE; HUMAN MILK; LACTIC ACID, L-; LACTOSE, UNSPECIFIED FORM; MATRICARIA RECUTITA; MENADIONE; OYSTER SHELL CALCIUM CARBONATE, CRUDE; PETROSELINUM CRISPUM; PHENYL ISOTHIOCYANATE; PIPERINE; QUERCETIN; RUTIN; SALMONELLA ENTERICA SUBSP. ENTERICA SEROVAR ENTERITIDIS; SKIM MILK; SUS SCROFA ILEUM; SUS SCROFA JEJUNUM; SUS SCROFA OVARY; SUS SCROFA STOMACH; TOXICODENDRON PUBESCENS LEAF; TRIBASIC CALCIUM PHOSPHATE (component of)
- Antimony potassium tartrate; arsenic trioxide; bryonia alba whole; daphne mezereum bark; echinacea angustifolia whole; felis catus milk; gelsemium sempervirens root; histamine dihydrochloride; hyoscyamus niger; influenza A virus A/tasmania/503/2020 ivr-221 (H3N2) antigen (formaldehyde inactivated); influenza A virus A/victoria/2570/2019 ivr-215 (H1N1) antigen (formaldehyde inactivated); influenza B virus B/phuket/3073/2013 antigen (formaldehyde inactivated); influenza B virus B/washington/02/2019 antigen (formaldehyde inactivated); mercurius solubilis; niacinamide; polygala senega root; pork liver; rhododendron tomentosum leafy twig; saccharomyces cerevisiae rna; silicon dioxide; spigelia anthelmia whole; sulfur; thuja occidentalis leafy twig; zinc (component of)
- Achillea millefolium; aconitum napellus; apis mellifera; arnica montana; arsenic trioxide; atropa belladonna; bellis perennis; bryonia alba root; calendula officinalis flowering top; citrullus colocynthis fruit pulp; clematis vitalba flower; comfrey root; echinacea angustifolia; echinacea purpurea; eupatorium perfoliatum flowering top; ferrosoferric phosphate; hamamelis virginiana root bark/stem bark; helianthemum nummularium flower; histamine dihydrochloride; hypericum perforatum; impatiens glandulifera flower; ledum palustre twig; matricaria chamomilla whole; mentha piperita; ornithogalum umbellatum flowering top; plantago major; prunus cerasifera flower; solanum dulcamara whole; sulfur; toxicodendron pubescens leaf; veratrum album root; verbascum thapsus (component of)
- Antimony potassium tartrate; arsenic trioxide; bryonia alba root; daphne mezereum bark; echinacea angustifolia whole; felis catus milk; gelsemium sempervirens root; histamine dihydrochloride; hyoscyamus niger; influenza A virus A/darwin/9/2021 ivr-228 (H3N2) antigen (UV, formaldehyde inactivated); influenza A virus A/victoria/2570/2019 ivr-215 (H1N1) antigen (UV, formaldehyde inactivated); influenza B virus B/austria/1359417/2021 bvr-26 antigen (UV, formaldehyde inactivated); influenza B virus B/phuket/3073/2013 antigen (UV, formaldehyde inactivated); mercurius solubilis; niacinamide; polygala senega root; pork liver; rhododendron tomentosum leafy twig; saccharomyces cerevisiae rna; silicon dioxide; spigelia anthelmia whole; sulfur; thuja occidentalis leafy twig; zinc (component of)
- Achillea millefolium; aconitum napellus whole; apis mellifera; arnica montana; arsenic trioxide; atropa belladonna; bellis perennis; bryonia alba root; calendula officinalis flowering top; citrullus colocynthis fruit pulp; clematis vitalba flower; comfrey root; echinacea angustifolia; echinacea purpurea; eupatorium perfoliatum flowering top; ferrosoferric phosphate; hamamelis virginiana root bark/stem bark; helianthemum nummularium flower; histamine dihydrochloride; hypericum perforatum; impatiens glandulifera flower; matricaria chamomilla; mentha piperita; ornithogalum umbellatum flowering top; plantago major; prunus cerasifera flower; rhododendron tomentosum leafy twig; solanum dulcamara whole; sulfur; toxicodendron pubescens leaf; veratrum album root; verbascum thapsus (component of)
- Amaranthus retroflexus pollen; ambrosia acanthicarpa pollen; ambrosia artemisiifolia pollen; ambrosia psilostachya pollen; ambrosia trifida pollen; anthoxanthum odoratum pollen; apis mellifera; berberis vulgaris root bark; bos taurus skin; canis lupus familiaris skin; capra hircus hair; chelidonium majus; chenopodium album pollen; cow milk; cynodon dactylon pollen; equus caballus skin; fagus sylvatica flower bud; felis catus hair; formic acid; histamine dihydrochloride; house dust; juniperus communis stem; luffa operculata fruit; monosodium glutamate; ovis aries skin; peanut; ribes nigrum flower bud; schoenocaulon officinale seed; sheep wool; solidago virgaurea flowering top; sorghum halepense pollen; taraxacum officinale; urtica urens; wheat; xanthium strumarium pollen (component of)
- ACTIVATED CHARCOAL; ALMOND; ANEMONE PRATENSIS; APPLE; ATLANTIC COD; ATLANTIC SALMON; BANANA; BEEF; BLACK PEPPER; BLACK WALNUT; BRAZIL NUT; CANOLA OIL; CASEIN, LACTOCOCCUS LACTIS CULTURED, PENICILLIUM ROQUEFORTI CULTURED, AGED; CASHEW; CHICKEN; COCOA; COCONUT; COFFEA ARABICA SEED, ROASTED; COMMON SHRIMP; CORTISONE ACETATE; COW MILK; EDIBLE ROCK CRAB; EGG; EGGPLANT; ENGLISH WALNUT; FERROUS IODIDE; FLAX SEED; GELSEMIUM SEMPERVIRENS ROOT; GREEN TEA LEAF; HISTAMINE DIHYDROCHLORIDE; HYALURONIDASE; INTERFERON gamma PORCINE RECOMBINANT; IODINE; IRIDIUM; IRON; KIDNEY BEAN; LAMB; LYCOPODIUM CLAVATUM SPORE; NORTHERN BLUEFIN TUNA; ONION; ORANGE; PACIFIC OYSTER; PEANUT; PECAN; PORK; PORK LIVER; POTASSIUM GLUCONATE; POTATO; SELENIUM; SEROTONIN HYDROCHLORIDE; SESAME SEED; STRAWBERRY; SUS SCROFA ADRENAL GLAND; TOMATO; WHEAT; ZINC (component of)
- ACTIVATED CHARCOAL; ANEMONE PRATENSIS; APPLE; ATLANTIC COD; ATLANTIC SALMON; BANANA; BEEF; BITTER ALMOND; BLACK WALNUT; BRAZIL NUT; CANOLA OIL; CASEIN, LACTOCOCCUS LACTIS CULTURED, PENICILLIUM ROQUEFORTI CULTURED, AGED; CASHEW; CHICKEN; COCOA; COCONUT; COFFEA ARABICA SEED, ROASTED; COMMON SHRIMP; CORTISONE ACETATE; COW MILK; EDIBLE ROCK CRAB; EGG; EGGPLANT; ENGLISH WALNUT; FERROUS IODIDE; FLAX SEED; FRAGARIA VESCA FRUIT; GELSEMIUM SEMPERVIRENS ROOT; GREEN PEPPERCORN; GREEN TEA LEAF; HISTAMINE DIHYDROCHLORIDE; HYALURONIDASE; INTERFERON gamma PORCINE RECOMBINANT; IODINE; IRIDIUM; IRON; KIDNEY BEAN; LAMB; LYCOPODIUM CLAVATUM SPORE; NORTHERN BLUEFIN TUNA; ONION; ORANGE; PACIFIC OYSTER; PEANUT; PECAN; PORK; PORK LIVER; POTASSIUM GLUCONATE; SELENIUM; SEROTONIN HYDROCHLORIDE; SESAME SEED; SOLANUM TUBEROSUM WHOLE; SUS SCROFA ADRENAL GLAND; TOMATO; WHEAT; ZINC (component of)


H315 (99%): Causes skin irritation [Warning Skin corrosion/irritation]
H317 (96.9%): May cause an allergic skin reaction [Warning Sensitization, Skin]
H319 (99%): Causes serious eye irritation [Warning Serious eye damage/eye irritation]
H334 (96.9%): May cause allergy or asthma symptoms or breathing difficulties if inhaled [Danger Sensitization, respiratory]
H335 (97.9%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation]
P233, P260, P261, P264, P264+P265, P271, P272, P280, P284, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P333+P317, P337+P317, P342+P316, P362+P364, P403, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 97 reports by companies from 16 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Reported as not meeting GHS hazard criteria per 1 of 97 reports by companies. For more detailed information, please visit ECHA C&L website.
There are 15 notifications provided by 96 of 97 reports by companies with hazard statement code(s).
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Skin Irrit. 2 (99%)
Skin Sens. 1B (96.9%)
Eye Irrit. 2 (99%)
Resp. Sens. 1B (96.9%)
STOT SE 3 (97.9%)
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=PPZMYIBUHIPZOS-UHFFFAOYSA-N
- Australian Industrial Chemicals Introduction Scheme (AICIS)1H-Imidazole-4-ethanamine, dihydrochloridehttps://services.industrialchemicals.gov.au/search-inventory/
- ChemIDplusHistamine dihydrochloride [USAN]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000056928ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA Chemicals under the TSCA1H-Imidazole-5-ethanamine, hydrochloride (1:2)https://www.epa.gov/chemicals-under-tscaEPA TSCA Classificationhttps://www.epa.gov/tsca-inventory
- EPA DSSTox1H-Imidazole-4-ethanamine, dihydrochloridehttps://comptox.epa.gov/dashboard/DTXSID1058767CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-notice2-imidazol-4-ylethylamine dihydrochloridehttps://chem.echa.europa.eu/100.000.2722-imidazol-4-ylethylamine dihydrochloride (EC: 200-298-4)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/127704
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingHISTAMINE DIHYDROCHLORIDEhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/3POA0Q644U
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/1H-Imidazole-4-ethanamine, dihydrochloridehttps://www.epa.govt.nz/industry-areas/hazardous-substances/guidance-for-importers-and-manufacturers/hazardous-substances-databases/
- CCSbaseCCSbase Classificationhttps://ccsbase.net/
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- DailyMedHISTAMINE DIHYDROCHLORIDEhttps://dailymed.nlm.nih.gov/dailymed/search.cfm?labeltype=all&query=HISTAMINE+DIHYDROCHLORIDE
- EU Clinical Trials Register
- European Medicines Agency (EMA)LICENSEInformation on the European Medicines Agency's (EMA) website is subject to a disclaimer and copyright and limited reproduction notices.https://www.ema.europa.eu/en/about-us/legal-noticeCeplene (EMEA/H/C/000796)https://www.ema.europa.eu/en/medicines/human/EPAR/ceplene
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licenceHISTAMINE DIHYDROCHLORIDEhttps://platform.opentargets.org/drug/CHEMBL1533310
- WHO Anatomical Therapeutic Chemical (ATC) ClassificationLICENSEUse of all or parts of the material requires reference to the WHO Collaborating Centre for Drug Statistics Methodology. Copying and distribution for commercial purposes is not allowed. Changing or manipulating the material is not allowed.https://www.whocc.no/copyright_disclaimer/Histamine dihydrochloridehttps://www.whocc.no/atc_ddd_index/?code=L03AX14
- Japan Pharmaceuticals and Medical Devices Agency (PMDA)Histamine dihydrochloridehttps://www.pmda.go.jp/english/review-services/reviews/approved-information/drugs/0001.html
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlTherapeutic category of drugs in Japanhttp://www.genome.jp/kegg-bin/get_htext?br08301.kegAnatomical Therapeutic Chemical (ATC) classificationhttp://www.genome.jp/kegg-bin/get_htext?br08303.keg
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- National Drug Code (NDC) DirectoryLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingHISTAMINE DIHYDROCHLORIDEhttps://www.fda.gov/drugs/drug-approvals-and-databases/national-drug-code-directory
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.htmlhistamine dihydrochloridehttps://rxnav.nlm.nih.gov/id/rxnorm/142136
- SpectraBaseHISTAMINE, DIHYDROCHLORIDEhttps://spectrabase.com/spectrum/KkEHfafIaZsHISTAMINE DIHYDROCHLORIDEhttps://spectrabase.com/spectrum/8kHGpoCKaudhistamine, dihydrochloridehttps://spectrabase.com/spectrum/BlprEV3bmm31H-imidazolium, 4-(2-ammonioethyl)-, dichloridehttps://spectrabase.com/spectrum/3x8WBxaJvwaHISTAMINE, DIHYDROCHLORIDEhttps://spectrabase.com/spectrum/BFHi8WCOnjeHistamine dihydrochloridehttps://spectrabase.com/spectrum/GndtpNq9LOYhistamine, dihydrochloridehttps://spectrabase.com/spectrum/6PSwrZMuji1HISTAMINIUM DICHLORIDEhttps://spectrabase.com/spectrum/6TBe44Ps5Ex1H-Imidazole-4-ethanamine, dihydrochloridehttps://spectrabase.com/spectrum/8scZ4NTgZPCHistamine, dihydrochloridehttps://spectrabase.com/spectrum/Jp5phMFVIFGHistamine, dihydrochloridehttps://spectrabase.com/spectrum/D25VAbQHviP
- Springer Nature
- Wikidatahistamine dihydrochloridehttps://www.wikidata.org/wiki/Q5772985
- WikipediaDioxosuccinic acidhttps://en.wikipedia.org/wiki/Dioxosuccinic_acidHistamine dihydrochloridehttps://en.wikipedia.org/wiki/Histamine_dihydrochloride
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlHistamine Agonistshttps://www.ncbi.nlm.nih.gov/mesh/68017442
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403381922https://pubchem.ncbi.nlm.nih.gov/substance/403381922
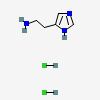

 CID 313 (Hydrochloric Acid)
CID 313 (Hydrochloric Acid)
