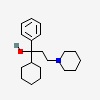
Trihexyphenidyl
- trihexyphenidyl
- Benzhexol
- 144-11-6
- Triphenidyl
- Trihexyphenidyle
- Create:2005-03-25
- Modify:2025-01-18
 Trihexyphenidyl Hydrochloride (has salt form);
Trihexyphenidyl Hydrochloride (has salt form);  Trihexyphenidyl, (R)- (annotation moved to).
Trihexyphenidyl, (R)- (annotation moved to).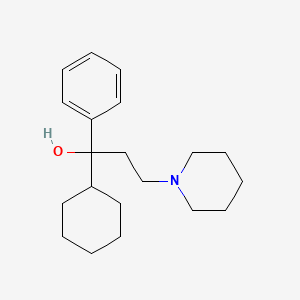
- Apo Trihex
- Apo-Trihex
- ApoTrihex
- Artane
- Benzhexol
- Cyclodol
- Hipokinon
- Parkinane
- Parkopan
- Trihexane
- Trihexidyl Hydrochloride
- Trihexyphenidyl
- Trihexyphenidyl Hydrochloride
- Trihexyphenidyl Hydrochloride Elixir
- trihexyphenidyl
- Benzhexol
- 144-11-6
- Triphenidyl
- Trihexyphenidyle
- Parkinane retard
- Trihexylphenedyl
- Benzhexolum
- Sedrena (free base)
- Trihexifenidilo
- Trihexyphenidylum
- Apo-Trihex
- Trihexifenidilo [INN-Spanish]
- Trihexyphenidyle [INN-French]
- Trihexyphenidylum [INN-Latin]
- 1-Piperidinepropanol, alpha-cyclohexyl-alpha-phenyl-
- Benzhexol free base
- 1-cyclohexyl-1-phenyl-3-(piperidin-1-yl)propan-1-ol
- HSDB 3196
- 1-cyclohexyl-1-phenyl-3-piperidin-1-ylpropan-1-ol
- UNII-6RC5V8B7PO
- EINECS 205-614-4
- 6RC5V8B7PO
- NSC 12268
- NSC-12268
- BRN 0088959
- Trihexyphenidyl (INN)
- 1-Piperidinepropanol, .alpha.-cyclohexyl-.alpha.-phenyl-
- CHEBI:9720
- 144-11-6 (free base)
- DTXSID4023705
- 5-20-02-00231 (Beilstein Handbook Reference)
- 1-cyclohexyl-1-phenyl-3-(1-piperidyl)propan-1-ol
- Trihexylphenidyl
- Trihexylphenizyl
- Trihexylphenidyle
- TRIHEXYPHENIDYL [INN]
- Trihexifenidilo (INN-Spanish)
- Trihexyphenidyle (INN-French)
- Trihexyphenidylum (INN-Latin)
- Trihexyphenidyl [INN:BAN]
- 1-Piperidinepropanol, alpha-cyclohexyl-alpha-phenyl-,
- Apo-trihex (TN)
- (+/-)-Triphenidyl; Benzhexol; NSC 12268
- Spectrum_000632
- Prestwick0_000701
- Prestwick1_000701
- Prestwick2_000701
- Prestwick3_000701
- Spectrum2_000829
- Spectrum3_001375
- Spectrum4_000369
- Spectrum5_001465
- CHEMBL1490
- Lopac0_001125
- Oprea1_853391
- SCHEMBL34645
- BSPBio_000881
- BSPBio_002930
- KBioGR_000837
- KBioSS_001112
- DivK1c_000372
- TRIHEXYPHENIDYL [HSDB]
- SPBio_000757
- SPBio_002802
- TRIHEXYPHENIDYL [VANDF]
- BPBio1_000971
- DTXCID703705
- GTPL7315
- TRIHEXYPHENIDYL [WHO-DD]
- BDBM81462
- KBio1_000372
- KBio2_001112
- KBio2_003680
- KBio2_006248
- KBio3_002150
- DTXSID40904831
- N04AA01
- NINDS_000372
- NSC_5572
- NSC12268
- STL300356
- AKOS015914081
- CCG-205200
- DB00376
- SDCCGSBI-0051093.P004
- IDI1_000372
- MRF-0000523
- NCGC00016003-03
- NCGC00016003-04
- NCGC00016003-06
- NCGC00016003-07
- NCGC00016003-15
- NCGC00089797-02
- ()-Triphenidyl; Benzhexol; NSC 12268
- DA-58731
- LS-14821
- SBI-0051093.P003
- CAS_58947-95-8
- AB00053559
- NS00006523
- C07171
- D08638
- EN300-708793
- G78220
- AB00053559_13
- alpha-Cyclohexyl-alpha-phenyl-1-piperidinepropanol
- L000821
- Q2991856
- BRD-A48180038-003-05-2
- BRD-A48180038-003-15-1
- BRD-A48180038-003-24-3
- .alpha.-Cyclohexyl-.alpha.-phenyl-1-piperidinepropanol
- 1-Cyclohexyl-1-phenyl-3-(1-piperidinyl)-1-propanol #
- (R)-1-cyclohexyl-1-phenyl-3-(piperidin-1-yl)propan-1-ol
173.72 Ų [M+H]+ [CCS Type: TW; Method: calibrated with polyalanine and drug standards]
169.71 Ų [M+H-H2O]+ [CCS Type: TW; Method: calibrated with polyalanine and drug standards]
178.37 Ų [M+Na]+ [CCS Type: TW; Method: calibrated with polyalanine and drug standards]
Antiseizure medicines
Medicines for parkinsonism
302.250946 100
303.252563 60.32
98.097282 58.54
284.237823 8.87
304.254333 8.54
222.1861 100
77.03955 14.40
137.09744 12.70
300.23519 4.30
222.18636 100
137.09711 63.10
187.11311 3.10
135.08171 1.30
300.23456 1.10
324.22968 100
106.06518 9.20
113.63924 1.20
59.22823 1.10
124.73866 1.10
Trihexyphenidyl Hydrochloride (has salt form)
Trihexyphenidyl, (R)- (annotation moved to)
Use (kg; approx.) in Germany (2009): >10
Use (kg) in France (2004): 257
Consumption (g per capita; approx.) in Germany (2009): 0.000122
Consumption (g per capita) in France (2004): 0.00425
Calculated removal (%): 72.7

P264, P270, P301+P316, P321, P330, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 2 reports by companies from 1 notifications to the ECHA C&L Inventory.
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
IMAP assessments - 1-Piperidinepropanol, .alpha.-cyclohexyl-.alpha.-phenyl-: Human health tier I assessment
IMAP assessments - 1-Piperidinepropanol, .alpha.-cyclohexyl-.alpha.-phenyl-: Environment tier I assessment
Trihexyphenidyl has not been reported to cause serum aminotransferase elevations, but it has not been evaluated for effects on serum enzyme levels in a prospective manner. Trihexyphenidyl was cited as the cause of two cases of acute liver injury resulting in death in the Japanese literature, but few details were given and there have been no other reports of such injury in the literature in the subsequent 40 years. Thus, trihexyphenidyl must be a very rare cause of liver injury, if it occurs at all.
Likelihood score: E* (unproven but suspected cause of clinically apparent liver injury).
M Chen, V Vijay, Q Shi, Z Liu, H Fang, W Tong. FDA-Approved Drug Labeling for the Study of Drug-Induced Liver Injury, Drug Discovery Today, 16(15-16):697-703, 2011. PMID:21624500 DOI:10.1016/j.drudis.2011.05.007
M Chen, A Suzuki, S Thakkar, K Yu, C Hu, W Tong. DILIrank: the largest reference drug list ranked by the risk for developing drug-induced liver injury in humans. Drug Discov Today 2016, 21(4): 648-653. PMID:26948801 DOI:10.1016/j.drudis.2016.02.015
◉ Summary of Use during Lactation
Limited information indicates that maternal doses of trihexyphenidyl up to 4 mg daily together with haloperidol or risperidone did not produce any adverse effects in breastfed infants. Long-term use of trihexyphenidyl might reduce milk production or milk letdown, but a single dose is not likely to interfere with breastfeeding. The prolactin elevating effect of concurrent antipsychotic agents might counteract any prolactin lowering effect of trihexyphenidyl. During long-term use, observe for signs of decreased lactation (e.g., infant insatiety and poor weight gain).
◉ Effects in Breastfed Infants
One woman with schizophrenia took trihexyphenidyl and haloperidol during 3 pregnancies and postpartum. The trihexyphenidyl dose was 4 mg daily in all 3 pregnancies. She breastfed (extent not stated) all 3 children for 6 to 8 months using the same doses. Development was age-appropriate in all children aged 16 months at 8 years of age at the time of assessment.
A woman diagnosed with undifferentiated schizophrenia took risperidone 4 to 5 mg and trihexyphenidyl 2 mg daily throughout 5 pregnancies. She breastfed each infant for 20 to 24 months. No adverse developmental consequences were noted in any of the children. At the time of publication, the oldest three children, aged 26, 23 and 22 years, had completed their education and were employed, while the youngest two were 15 and 19 years old and were doing well academically in their education.
◉ Effects on Lactation and Breastmilk
Anticholinergics can inhibit lactation in animals, apparently by inhibiting growth hormone and oxytocin secretion. Anticholinergic drugs can also reduce serum prolactin in nonnursing women. The prolactin level in a mother with established lactation may not affect her ability to breastfeed.
One woman with schizophrenia took trihexyphenidyl and haloperidol during 3 pregnancies and postpartum. She was able to breastfeed (extent not stated) all 3 children for 6 to 8 months.
A woman diagnosed with undifferentiated schizophrenia took risperidone 4 to 5 mg and trihexyphenidyl 2 mg daily throughout 5 pregnancies. She successfully breastfed each infant for 20 to 24 months.
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=HWHLPVGTWGOCJO-UHFFFAOYSA-N
- Australian Industrial Chemicals Introduction Scheme (AICIS)1-Piperidinepropanol, .alpha.-cyclohexyl-.alpha.-phenyl-https://services.industrialchemicals.gov.au/search-assessments/
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/(±)-Trihexyphenidylhttps://commonchemistry.cas.org/detail?cas_rn=144-11-6
- ChemIDplusTrihexyphenidyl [INN:BAN]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000144116ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_useTrihexyphenidylhttps://www.drugbank.ca/drugs/DB00376
- DTP/NCILICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuse
- EPA DSSToxTrihexyphenidylhttps://comptox.epa.gov/dashboard/DTXSID4023705(S)-Trihexyphenidylhttps://comptox.epa.gov/dashboard/DTXSID40904831CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeTrihexyphenidylhttps://chem.echa.europa.eu/100.005.105Trihexyphenidyl (EC: 205-614-4)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/12799
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Hazardous Substances Data Bank (HSDB)TRIHEXYPHENIDYLhttps://pubchem.ncbi.nlm.nih.gov/source/hsdb/3196
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingTrihexyphenidylhttp://www.hmdb.ca/metabolites/HMDB0014520HMDB0014520_msms_450464https://hmdb.ca/metabolites/HMDB0014520#spectra
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/1-Piperidinepropanol, .alpha.-cyclohexyl-.alpha.-phenyl-https://www.epa.govt.nz/industry-areas/hazardous-substances/guidance-for-importers-and-manufacturers/hazardous-substances-databases/
- CCSbaseCCSbase Classificationhttps://ccsbase.net/
- ChEBITrihexyphenidylhttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:9720
- LiverTox
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licenceTRIHEXYPHENIDYLhttps://platform.opentargets.org/drug/CHEMBL1490
- Toxin and Toxin Target Database (T3DB)LICENSET3DB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (T3DB) and the original publication.http://www.t3db.ca/downloadsTrihexyphenidylhttp://www.t3db.ca/toxins/T3D2770
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jspTrihexyphenidylhttps://ctdbase.org/detail.go?type=chem&acc=D014282
- Drug Gene Interaction database (DGIdb)LICENSEThe data used in DGIdb is all open access and where possible made available as raw data dumps in the downloads section.http://www.dgidb.org/downloadsTRIHEXYPHENIDYLhttps://www.dgidb.org/drugs/rxcui:10811
- IUPHAR/BPS Guide to PHARMACOLOGYLICENSEThe Guide to PHARMACOLOGY database is licensed under the Open Data Commons Open Database License (ODbL) https://opendatacommons.org/licenses/odbl/. Its contents are licensed under a Creative Commons Attribution-ShareAlike 4.0 International License (http://creativecommons.org/licenses/by-sa/4.0/)https://www.guidetopharmacology.org/about.jsp#licenseGuide to Pharmacology Target Classificationhttps://www.guidetopharmacology.org/targets.jsp
- Therapeutic Target Database (TTD)Trihexyphenidylhttps://idrblab.net/ttd/data/drug/details/D09GFL
- Drug Induced Liver Injury Rank (DILIrank) DatasetLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Drugs and Lactation Database (LactMed)Trihexyphenidylhttps://www.ncbi.nlm.nih.gov/books/n/lactmed/LM769/
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/TRIHEXYPHENIDYLNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- WHO Model Lists of Essential MedicinesLICENSEPermission from WHO is not required for the use of WHO materials issued under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Intergovernmental Organization (CC BY-NC-SA 3.0 IGO) license.https://www.who.int/about/policies/publishing/copyrightTrihexyphenidylhttps://list.essentialmeds.org/medicines/410
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawTrihexyphenidylhttp://www.nist.gov/srd/nist1a.cfm
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlAnatomical Therapeutic Chemical (ATC) classificationhttp://www.genome.jp/kegg-bin/get_htext?br08303.kegTarget-based classification of drugshttp://www.genome.jp/kegg-bin/get_htext?br08310.keg
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- Metabolomics Workbench
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- SpectraBaseTrihexyphenidylhttps://spectrabase.com/spectrum/7V59so77RS2Trihexyphenidylhttps://spectrabase.com/spectrum/JeW8FTwkB8rDL-Trihexyphenidylhttps://spectrabase.com/spectrum/EFN0yXXC68TRIHEXPHENIDYL HYDROCHLORIDEhttps://spectrabase.com/spectrum/7K2kDo9I1um
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.htmltrihexyphenidylhttps://rxnav.nlm.nih.gov/id/rxnorm/10811
- WHO Anatomical Therapeutic Chemical (ATC) ClassificationLICENSEUse of all or parts of the material requires reference to the WHO Collaborating Centre for Drug Statistics Methodology. Copying and distribution for commercial purposes is not allowed. Changing or manipulating the material is not allowed.https://www.whocc.no/copyright_disclaimer/Trihexyphenidylhttps://www.whocc.no/atc_ddd_index/?code=N04AA01
- PharmGKBLICENSEPharmGKB data are subject to the Creative Commons Attribution-ShareALike 4.0 license (https://creativecommons.org/licenses/by-sa/4.0/).https://www.pharmgkb.org/page/policiestrihexyphenidylhttps://www.pharmgkb.org/chemical/PA164747026
- PharosLICENSEData accessed from Pharos and TCRD is publicly available from the primary sources listed above. Please respect their individual licenses regarding proper use and redistribution.https://pharos.nih.gov/abouttrihexyphenidylhttps://pharos.nih.gov/ligands/T26WWR711LGY
- Springer Nature
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidatatrihexyphenidylhttps://www.wikidata.org/wiki/Q2991856
- WikipediaDihomo-γ-linolenic acidhttps://en.wikipedia.org/wiki/Dihomo-γ-linolenic_acidTrihexyphenidylhttps://en.wikipedia.org/wiki/Trihexyphenidyl
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlTrihexyphenidylhttps://www.ncbi.nlm.nih.gov/mesh/68014282Antiparkinson Agentshttps://www.ncbi.nlm.nih.gov/mesh/68000978Muscarinic Antagonistshttps://www.ncbi.nlm.nih.gov/mesh/68018727
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403464278https://pubchem.ncbi.nlm.nih.gov/substance/403464278
- NCBI