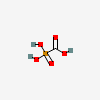
Foscarnet
- foscarnet
- Phosphonoformic acid
- Phosphonoformate
- Carboxyphosphonic acid
- 4428-95-9
- Create:2005-03-25
- Modify:2025-01-18
Foscarnet sodium is an antiviral prescription medicine approved by the U.S. Food and Drug Administration (FDA) for the treatment of cytomegalovirus (CMV) retinitis in individuals with AIDS. Foscarnet sodium is also FDA-approved for the treatment of a certain type of herpes simplex virus (HSV)infection, called acyclovir-resistant mucocutaneous HSV infection, in people with weakened immune systems.
CMV diseases—such as those affecting the eye (retinitis)—and HSV diseases can be opportunistic infections (OIs) of HIV.
 Foscarnet Sodium (has salt form).
Foscarnet Sodium (has salt form).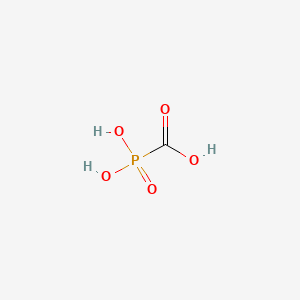
- Foscarnet
- Foscarnet Barium (2:3) Salt
- Foscarnet Calcium (2:3) Salt
- Foscarnet Disodium Salt
- Foscarnet Magnesium (2:3) Salt
- Foscarnet Manganese (2+) (2:3) Salt
- Foscarnet Sodium
- Foscarnet Sodium Hexahydrate
- Foscarnet Trilithium Salt
- Foscarnet Tripotassium Salt
- Foscarnet Trisodium Salt
- Foscavir
- Phosphonoformate
- Phosphonoformic Acid
- Trisodium Phosphonoformate
- foscarnet
- Phosphonoformic acid
- Phosphonoformate
- Carboxyphosphonic acid
- 4428-95-9
- Foscarmet
- Foscavir
- phosphonocarboxylic acid
- phosphonomethanoic acid
- Dihydroxyphosphinecarboxylic acid oxide
- Formic acid, phosphono-
- UNII-364P9RVW4X
- 364P9RVW4X
- HSDB 8122
- CHEBI:127780
- Phosphinecarboxylic acid, dihydroxy-, oxide
- dihydroxyphosphanecarboxylic acid oxide
- CHEMBL666
- DTXSID0048478
- J05AD01
- Phgosphonocarboxylic acid
- NSC313410
- Forscarnet
- 1nki
- Foscarnet (PFA)
- Spectrum_001359
- PFA & rIFN.alpha.A
- Spectrum2_000668
- Spectrum3_001484
- Spectrum4_000840
- Spectrum5_000932
- FOSCARNET [VANDF]
- phosphonoformic acid(PFA)
- MSL & PFA
- FOSCARNET [WHO-DD]
- Phosphono-formic acid(PFA)
- hydroxycarbonylphosphonic acid
- SCHEMBL23633
- BSPBio_003067
- Foscarnet & IFN-.ALPHA.
- KBioGR_001419
- KBioSS_001839
- DivK1c_000915
- SPBio_000735
- GTPL5497
- DTXCID0028452
- KBio1_000915
- KBio2_001839
- KBio2_004407
- KBio2_006975
- KBio3_002567
- NINDS_000915
- ZJAOAACCNHFJAH-UHFFFAOYSA-N
- BDBM50011181
- Phosphonoformic acid & IFN-.ALPHA.
- AKOS006281397
- DB00529
- IDI1_000915
- (PFA)dihydroxyphosphinecarboxylic acid oxide
- dihydroxyphosphinecarboxylic acid oxide(PFA)
- NS00005428
- C06456
- EN300-708767
- Q420387
- BRD-K85146014-314-05-4
- BRD-K85146014-314-06-2
- BRD-K85146014-314-07-0
- Phosphonoformate(trisodium) & Recombinant Alpha-A Interferon
- Dihydroxyphosphinecarboxylic acid oxide and MSL, neutralizing monoclonal antibody
 Foscarnet Sodium (has salt form)
Foscarnet Sodium (has salt form)Foscarnet sodium is an antiviral prescription medicine approved by the U.S. Food and Drug Administration (FDA) for the treatment of cytomegalovirus (CMV) retinitis in individuals with AIDS. Foscarnet sodium is also FDA-approved for the treatment of a certain type of herpes simplex virus (HSV)infection, called acyclovir-resistant mucocutaneous HSV infection, in people with weakened immune systems.
CMV diseases—such as those affecting the eye (retinitis)—and HSV diseases can be opportunistic infections (OIs) of HIV.
2.13 +/- 0.71 mL/min/kg [patients had normal renal function (CrCl > 80 mL/min]
68 +/- 8 mL/min/kg [CrCl was 50-80 mL/min]
34 +/- 9 mL/min/kg [CrCl was 25-49 mL/min]
20 +/- 4 mL/min/kg [CrCl was 10 - 24 mL/min]
- Cytoplasm
- Membrane

H341 (100%): Suspected of causing genetic defects [Warning Germ cell mutagenicity]
H373 (100%): May causes damage to organs through prolonged or repeated exposure [Warning Specific target organ toxicity, repeated exposure]
P203, P260, P280, P318, P319, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Muta. 2 (100%)
STOT RE 2 (100%)
Intravenous foscarnet therapy is associated with mild-to-moderate serum ALT elevations in a proportion of patients, but the drug is usually given to patients with multiorgan disease and conditions that may be associated with some degree of hepatic injury. The ALT elevations are usually asymptomatic and resolve even with continuation of foscarnet. Single case reports of clinically apparent liver injury that were possibly attributable to foscarnet have been reported. The pattern of injury was cholestatic arising within weeks of starting foscarnet but the clinical features of the liver injury have not been described in any detail.
Likelihood score: D (possible cause of clinically apparent liver injjry).
M Chen, V Vijay, Q Shi, Z Liu, H Fang, W Tong. FDA-Approved Drug Labeling for the Study of Drug-Induced Liver Injury, Drug Discovery Today, 16(15-16):697-703, 2011. PMID:21624500 DOI:10.1016/j.drudis.2011.05.007
M Chen, A Suzuki, S Thakkar, K Yu, C Hu, W Tong. DILIrank: the largest reference drug list ranked by the risk for developing drug-induced liver injury in humans. Drug Discov Today 2016, 21(4): 648-653. PMID:26948801 DOI:10.1016/j.drudis.2016.02.015
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=ZJAOAACCNHFJAH-UHFFFAOYSA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/
- ChemIDplusChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_use
- EPA DSSToxCompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticePhosphonocarboxylic acidhttps://echa.europa.euPhosphonocarboxylic acid (EC: 112-287-0)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/398259
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Hazardous Substances Data Bank (HSDB)
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citing
- CCSbaseCCSbase Classificationhttps://ccsbase.net/
- ChEBIPhosphonoformic acidhttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:127780
- Drug Database, Clinicalinfo.hiv.govLICENSEUnless otherwise noted, material presented on the HIV.gov website is considered Federal government information and is in the public domain. That means this information may be freely copied and distributed. We request that you use appropriate attribution to HIV.gov.https://www.hiv.gov/about-us/mission-and-teamFoscarnet Sodiumhttps://clinicalinfo.hiv.gov/en/drugs/foscarnet-sodium/patient
- FDA Pharm ClassesLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingFDA Pharmacological Classificationhttps://www.fda.gov/ForIndustry/DataStandards/StructuredProductLabeling/ucm162549.htm
- LiverTox
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/LOTUS Treehttps://lotus.naturalproducts.net/
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licence
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jsp
- Drug Gene Interaction database (DGIdb)LICENSEThe data used in DGIdb is all open access and where possible made available as raw data dumps in the downloads section.http://www.dgidb.org/downloads
- Drug Induced Liver Injury Rank (DILIrank) DatasetLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Drugs@FDALICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- EU Clinical Trials Register
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlAnatomical Therapeutic Chemical (ATC) classificationhttp://www.genome.jp/kegg-bin/get_htext?br08303.keg
- KNApSAcK Species-Metabolite DatabasePhosphonoformic acidhttp://www.knapsackfamily.com/knapsack_core/info.php?sname=C_ID&word=C00000800
- Metabolomics Workbench
- Nature Chemical Biology
- NIPH Clinical Trials Search of Japan
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.html
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/FOSCARNET SODIUMNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- WHO Anatomical Therapeutic Chemical (ATC) ClassificationLICENSEUse of all or parts of the material requires reference to the WHO Collaborating Centre for Drug Statistics Methodology. Copying and distribution for commercial purposes is not allowed. Changing or manipulating the material is not allowed.https://www.whocc.no/copyright_disclaimer/
- PharmGKBLICENSEPharmGKB data are subject to the Creative Commons Attribution-ShareALike 4.0 license (https://creativecommons.org/licenses/by-sa/4.0/).https://www.pharmgkb.org/page/policies
- Protein Data Bank in Europe (PDBe)
- RCSB Protein Data Bank (RCSB PDB)LICENSEData files contained in the PDB archive (ftp://ftp.wwpdb.org) are free of all copyright restrictions and made fully and freely available for both non-commercial and commercial use. Users of the data should attribute the original authors of that structural data.https://www.rcsb.org/pages/policies
- SpectraBasePHOSPHONOFORMIC-ACIDhttps://spectrabase.com/spectrum/5aT4QtmTc93PHOSPHONOFORMIC-ACIDhttps://spectrabase.com/spectrum/DaF1j5Tpv3Y
- Springer Nature
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidata
- WikipediaStrontium hexaboridehttps://en.wikipedia.org/wiki/Strontium_hexaboride
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlReverse Transcriptase Inhibitorshttps://www.ncbi.nlm.nih.gov/mesh/68018894Antiviral Agentshttps://www.ncbi.nlm.nih.gov/mesh/68000998
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403033978https://pubchem.ncbi.nlm.nih.gov/substance/403033978
- NCBI