Fludarabine Phosphate
- fludarabine phosphate
- 75607-67-9
- fludara
- Fludarabine 5'-monophosphate
- Oforta
- Create:2005-06-24
- Modify:2025-01-25
 Fludarabine (has active moiety).
Fludarabine (has active moiety).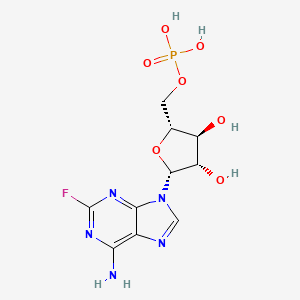
- 9 beta-D-arabinofuranosyl-2-fluoroadenine monophosphate
- 9H-purin-6-amine, 2-fluoro-9-(5-O-phosphono-beta-D-arabinofuranosyl)-
- Beneflur
- F-ara-AMP
- FaraAMP
- Fludara
- fludarabine 5'-monophosphate
- fludarabine monophosphate
- fludarabine phosphate
- fluoro-ara-AMP
- NSC 312887
- NSC-312887
- fludarabine phosphate
- 75607-67-9
- fludara
- Fludarabine 5'-monophosphate
- Oforta
- Fludarabine monophosphate
- 2-Fluoro-ARA AMP
- 2-F-ara-AMP
- Fludarabine (phosphate)
- FAMP
- NSC-312887
- CHEBI:63599
- UNII-1X9VK9O1SC
- 2-Fluoroadenine arabinoside 5'-monophosphate
- ((2R,3S,4S,5R)-5-(6-amino-2-fluoro-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl dihydrogen phosphate
- 1X9VK9O1SC
- 2-Fluoro-9-(5-O-phosphono-beta-D-arabinofuranosyl)-9H-purin-6-amine
- 2F-ara-AMP
- 9-beta-Arabinofuranosyl-2-fluoroadenine-5'-phosphate
- 9-beta-D-Arabinofuranosyl-2-fluoroadenine 5'-monophosphate
- MFCD00866418
- NSC-328002
- 9-beta-D-Arabinofuranosyl-2-fluoroadenine 5'-(dihydrogen phosphate)
- {[(2R,3S,4S,5R)-5-(6-AMINO-2-FLUORO-9H-PURIN-9-YL)-3,4-DIHYDROXYOXOLAN-2-YL]METHOXY}PHOSPHONIC ACID
- DTXSID2023060
- 75607-67-9 (phosphate)
- 9H-Purin-6-amine, 2-fluoro-9-(5-O-phosphono-b-D-arabinofuranosyl)-
- Fludarabine Phosphate (Fludara)
- Fludarabine phosphate [USAN:USP:BAN]
- NSC 312887
- NSC 328002
- [(2R,3S,4S,5R)-5-(6-amino-2-fluoropurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl dihydrogen phosphate
- FLUDARABINE PHOSPHATE (MART.)
- FLUDARABINE PHOSPHATE [MART.]
- FLUDARABINE PHOSPHATE (USP-RS)
- FLUDARABINE PHOSPHATE [USP-RS]
- FaraAMP
- fluoro-ara-AMP
- Fludarabine phosphate (USAN:USP:BAN)
- Fludarabinephosphate
- SMR002544683
- FLUDARABINE PHOSPHATE (EP MONOGRAPH)
- FLUDARABINE PHOSPHATE [EP MONOGRAPH]
- F-ara-AMP
- FLUDARABINE PHOSPHATE (USP MONOGRAPH)
- FLUDARABINE PHOSPHATE [USP MONOGRAPH]
- SR-05000001945
- Beneflur
- NSC312887
- 9H-Purin-6-amine, 2-fluoro-9-(5-O-phosphono-.beta.-D-arabinofuranosyl)-
- Fludarabine phosphate; 2-Fluoro-9-(5-O-phosphono-ss-D-arabinofuranosyl)-9H-purin-6-amine
- Fludara (TN)
- fludarabine-phosphate
- 2-Fluoro-ara-AMP
- Fludarabine phosphate?
- SCHEMBL3511
- MLS003915617
- MLS004774150
- Fludarabine Phosphate(Fludara)
- DTXCID103060
- 9 beta-D-arabinofuranosyl-2-fluoroadenine monophosphate
- 9-.beta.-D-Arabinofuranosyl-2-fluoroadenine 5'-(dihydrogen phosphate)
- CHEMBL1096882
- HY-B0028R
- Fludarabine phosphate (JAN/USP)
- SHT-586
- Fludarabine for system suitability
- GIUYCYHIANZCFB-FJFJXFQQSA-N
- GLXC-03004
- HMS2094O11
- Pharmakon1600-01505705
- FLUDARABINE PHOSPHATE [JAN]
- EX-A2028
- Fludarabine (phosphate) (Standard)
- HY-B0028
- FLUDARABINE PHOSPHATE [USAN]
- BDBM50248004
- FLUDARABINE PHOSPHATE [VANDF]
- HG1010
- NSC759194
- s1229
- 9H-Purin-6-amine, 2-fluoro-9-(5-O-phosphono-beta-D-arabinofuranosyl)-
- AKOS024464516
- FLUDARABINE PHOSPHATE [WHO-DD]
- BCP9000694
- CCG-213521
- CS-0861
- NSC-759194
- SRI-5907-04
- SRI-5907_05
- SRI-5907_07
- NCGC00182047-14
- AS-14202
- BCP0726000268
- FLUDARABINE 5'-MONOPHOSPHATE [MI]
- FLUDARABINE PHOSPHATE [ORANGE BOOK]
- SBI-0206893.P001
- SW218146-2
- D01907
- EN300-269343
- Q185916
- SR-05000001945-1
- SR-05000001945-4
- BRD-K71106091-001-05-3
- BRD-K71106091-001-06-1
- BRD-K71106091-001-09-5
- Z2235802254
- 9-beta-D-Arabinofuranosyl-2-fluoroadenine-5'-monophosphate
- [rac-(2R,3S,4S,5R)-5-(6-amino-2-fluoro-purin-9-yl)-3,4-dihydroxy-tetrahydrofuran-2-yl]methyl dihydrogen phosphate
 Fludarabine (has active moiety)
Fludarabine (has active moiety)Fludarabine phosphate is approved to treat adults with:
• B-cell chronic lymphocytic leukemia (CLL). It is used as part of combination therapy for B-cell CLL or alone for B-cell ALL that did not get better or got worse during at least one other treatment that included an alkylating agent.
Fludarabine phosphate is also being studied in the treatment of other types of cancer.
- Cytoplasm
- Membrane

H341 (97.5%): Suspected of causing genetic defects [Warning Germ cell mutagenicity]
H350 (29.6%): May cause cancer [Danger Carcinogenicity]
H360 (33.3%): May damage fertility or the unborn child [Danger Reproductive toxicity]
H361 (66.7%): Suspected of damaging fertility or the unborn child [Warning Reproductive toxicity]
H372 (29.6%): Causes damage to organs through prolonged or repeated exposure [Danger Specific target organ toxicity, repeated exposure]
P203, P260, P264, P270, P280, P318, P319, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 81 reports by companies from 7 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Muta. 2 (97.5%)
Carc. 1B (29.6%)
Repr. 1B (33.3%)
Repr. 2 (66.7%)
STOT RE 1 (29.6%)
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=GIUYCYHIANZCFB-FJFJXFQQSA-N
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/Fludarabine phosphatehttps://commonchemistry.cas.org/detail?cas_rn=75607-67-9
- ChemIDplusFludarabine phosphate [USAN:USP:BAN]https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0075607679ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DTP/NCILICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuse
- EPA DSSToxFludarabine phosphatehttps://comptox.epa.gov/dashboard/DTXSID2023060CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-notice9-bata-D-Arabinofuranosyl-2-fluoroadenine phosphatehttps://echa.europa.eu/substance-information/-/substanceinfo/100.123.7039-bata-D-Arabinofuranosyl-2-fluoroadenine phosphate (EC: 616-242-0)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/86795
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingFLUDARABINE PHOSPHATEhttps://gsrs.ncats.nih.gov/ginas/app/beta/substances/1X9VK9O1SC
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingFludarabinehttp://www.hmdb.ca/metabolites/HMDB0015206
- CCSbaseCCSbase Classificationhttps://ccsbase.net/
- ChEBIFludarabine phosphatehttps://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:63599
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licenceFLUDARABINE PHOSPHATEhttps://platform.opentargets.org/drug/CHEMBL1096882
- Toxin and Toxin Target Database (T3DB)LICENSET3DB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (T3DB) and the original publication.http://www.t3db.ca/downloadsFludarabinehttp://www.t3db.ca/toxins/T3D4736
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- DailyMed
- Drugs@FDALICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingFLUDARABINE PHOSPHATEhttps://www.accessdata.fda.gov/scripts/cder/daf/
- EU Clinical Trials Register
- FDA Orange BookLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- NCI Investigational DrugsFLUDARABINE PHOSPHATEhttp://dtp.nci.nih.gov/NCI-InvestigationalDrugsCI92/312887%20(1992).txt
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.htmlTherapeutic category of drugs in Japanhttp://www.genome.jp/kegg-bin/get_htext?br08301.kegUSP drug classificationhttp://www.genome.jp/kegg-bin/get_htext?br08302.kegAnatomical Therapeutic Chemical (ATC) classificationhttp://www.genome.jp/kegg-bin/get_htext?br08303.kegTarget-based classification of drugshttp://www.genome.jp/kegg-bin/get_htext?br08310.keg
- National Drug Code (NDC) DirectoryLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingFLUDARABINE PHOSPHATEhttps://www.fda.gov/drugs/drug-approvals-and-databases/national-drug-code-directory
- NCI Cancer DrugsFludarabine Phosphatehttps://www.cancer.gov/about-cancer/treatment/drugs/fludarabinephosphate
- NIPH Clinical Trials Search of Japan
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.htmlfludarabine phosphatehttps://rxnav.nlm.nih.gov/id/rxnorm/25102
- Therapeutic Target Database (TTD)Fludarabine phosphatehttps://idrblab.net/ttd/data/drug/details/D0TO2R
- PharmGKBLICENSEPharmGKB data are subject to the Creative Commons Attribution-ShareALike 4.0 license (https://creativecommons.org/licenses/by-sa/4.0/).https://www.pharmgkb.org/page/policiesfludarabinehttps://www.pharmgkb.org/chemical/PA449655
- PharosLICENSEData accessed from Pharos and TCRD is publicly available from the primary sources listed above. Please respect their individual licenses regarding proper use and redistribution.https://pharos.nih.gov/aboutfludarabine phosphatehttps://pharos.nih.gov/ligands/AH9PTBGQAMQQ
- Springer Nature
- Wikidatafludarabine phosphatehttps://www.wikidata.org/wiki/Q185916
- PubChemPFAS and Fluorinated Compounds in PubChemhttps://gitlab.com/uniluxembourg/lcsb/eci/pubchem-docs/-/raw/main/pfas-tree/PFAS_Tree.pdf?inline=false
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlfludarabine phosphatehttps://www.ncbi.nlm.nih.gov/mesh/67042382Antimetabolites, Antineoplastichttps://www.ncbi.nlm.nih.gov/mesh/68000964Immunosuppressive Agentshttps://www.ncbi.nlm.nih.gov/mesh/68007166
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 388488795https://pubchem.ncbi.nlm.nih.gov/substance/388488795
- NCBI

