Ferric chromate(VI)
- Ferric chromate
- 10294-52-7
- Ferric chromate(VI)
- Diiron tris(chromate)
- FERRIC CHROMATE, BASIC
- Create:2007-12-05
- Modify:2025-01-18
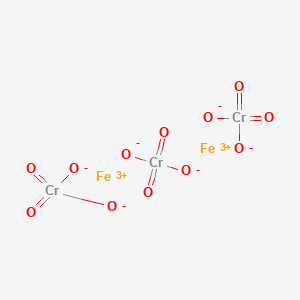
- Ferric chromate
- 10294-52-7
- Ferric chromate(VI)
- Diiron tris(chromate)
- FERRIC CHROMATE, BASIC
- UNII-O2123EJH4E
- O2123EJH4E
- dioxido(dioxo)chromium;iron(3+)
- EINECS 233-661-0
- Chromic acid (H2CrO4), iron (3+) salt (3:2)
- FERRIC CHROMATE(VI) [MI]
- Chromic acid (H2CrO4) iron(3+) salt (3:2)
- SIDERIN YELLOW
- IRON(III) CHROMATE
- FERRIC CHROMATE,BASIC
- Iron chromate (Fe2(CrO4)3)
- DTXSID101045361
- C.I. 77505
- Q4824655
Painting (Pigments, Binders, and Biocides) [Category: Paint]
Glass Manufacturing [Category: Industry]
Ceramics making [Category: Hobbies]
Enameling [Category: Hobbies]


H302: Harmful if swallowed [Warning Acute toxicity, oral]
H317: May cause an allergic skin reaction [Warning Sensitization, Skin]
H331: Toxic if inhaled [Danger Acute toxicity, inhalation]
H350: May cause cancer [Danger Carcinogenicity]
H373: May causes damage to organs through prolonged or repeated exposure [Warning Specific target organ toxicity, repeated exposure]
P203, P260, P261, P264, P270, P271, P272, P280, P301+P317, P302+P352, P304+P340, P316, P318, P319, P321, P330, P333+P317, P362+P364, P403+P233, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Carcinogenicity - category 1A
Specific target organ toxicity (repeated exposure) - category 2
Skin sensitisation - category 1
Acute toxicity (ingestion) - category 4
Acute toxicity (inhalation) - category 3
Occupational hepatotoxin - Secondary hepatotoxins: the potential for toxic effect in the occupational setting is based on cases of poisoning by human ingestion or animal experimentation.
Nephrotoxin - The chemical is potentially toxic to the kidneys in the occupational setting.
Dermatotoxin - Skin burns.
Skin Sensitizer - An agent that can induce an allergic reaction in the skin.
Asthma - Reversible bronchoconstriction (narrowing of bronchioles) initiated by the inhalation of irritating or allergenic agents.
IARC Carcinogen - Class 1: International Agency for Research on Cancer classifies chemicals as established human carcinogens.
NTP Carcinogen - Known to be a human carcinogen.
ACGIH Carcinogen - Confirmed Human.
Asthma, occupational [Category: Airway Disease]
Chromium, chronic toxic effect [Category: Metal Poisoning, Occupational]
Contact dermatitis, allergic [Category: Skin Disease]
- Australian Industrial Chemicals Introduction Scheme (AICIS)Chromic acid (H2CrO4), iron(3+) salt (3:2)https://services.industrialchemicals.gov.au/search-assessments/Chromic acid (H2CrO4), iron(3+) salt (3:2)https://services.industrialchemicals.gov.au/search-inventory/
- ChemIDplusFerric chromate(VI)https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0010294527ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- EPA DSSToxIron chromate (Fe2(CrO4)3)https://comptox.epa.gov/dashboard/DTXSID101045361CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeDiiron tris(chromate)https://echa.europa.eu/substance-information/-/substanceinfo/100.030.588
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Haz-Map, Information on Hazardous Chemicals and Occupational DiseasesLICENSECopyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://haz-map.com/AboutFerric chromate(VI)https://haz-map.com/Agents/8559
- Hazardous Chemical Information System (HCIS), Safe Work Australia
- Wikidataferric chromatehttps://www.wikidata.org/wiki/Q4824655
- WikipediaIron(III) chromatehttps://en.wikipedia.org/wiki/Iron(III)_chromate
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
 CID 23925 (Iron)
CID 23925 (Iron) CID 24425 (Chromic acid)
CID 24425 (Chromic acid)